Budesonide for chronic asthma in children and adults
- PMID: 11687183
- PMCID: PMC8713085
- DOI: 10.1002/14651858.CD003274
Budesonide for chronic asthma in children and adults
Abstract
Background: Inhaled budesonide is a widely used inhaled corticosteroid for asthma.
Objectives: The objectives of this review was to compare the efficacy of budesonide with placebo in the treatment of chronic asthma.
Search strategy: The Cochrane Airways Group Trial Register and reference lists of articles was searched. We contacted trialists for additional studies and searched abstracts of major respiratory society meetings (1997-1999).
Selection criteria: Randomised trials in children and adults comparing budesonide to placebo in the treatment of chronic asthma.
Data collection and analysis: Two reviewers independently assessed articles for inclusion and methodological quality. One reviewer extracted data.
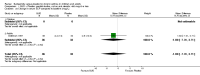
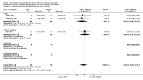
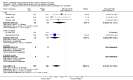
Main results: 43 studies met the inclusion criteria (2801 subjects). In non-oral steroid treated asthmatics, budesonide led to significant improvements in a number of measures of airway function. These included FEV1, Weighted Mean Difference (WMD) 3.7% predicted (95% CI 0.1, 7.2%); improvement in morning peak flow (PEF) from baseline WMD 29 L/min (95% CI 22, 36 L/min); improvement in evening PEF from baseline WMD 21 L/min (95% CI 13, 29 L/min). Varying methods of reporting symptoms limited the pooling of studies but all high methodological quality studies demonstrated significant improvements compared to placebo. Health status was not reported. Risk of trial withdrawal due to asthma exacerbation was lower with budesonide compared to placebo, relative risk 0.17 (95% CI 0.09, 0.33). Doses of 500-800 mcg/d appeared to have slightly larger effect sizes than lower doses, but no advantage for high doses were apparent. A single high quality RCT reported significant reductions in daily prednisolone requirement and the number of patients able to discontinue prednisolone completely in budesonide treated subjects compared to placebo. No difference in risk of oropharyngeal soreness/hoarseness or oral Candidiasis was apparent for budesonide compared to placebo. Long-term risk of adrenal insufficiency was not reported.
Reviewer's conclusions: This review strongly supports use of budesonide in chronic asthma. Consensus guidelines for chronic asthma suggest titrating inhaled steroid dose to individual requirements. Evidence from this review of trials does not present a case for routine dose titration above 800 mcg/d.
Conflict of interest statement
None
Figures





























































































































































References
References to studies included in this review
Aaronson 1998 {published data only}
-
- Aaronson D, Kaiser H, Dockhorn R, Findlay S, Korenblat P, Thorsson L, et al. Effects of budesonide by means of the Turbuhaler on the hypothalmic‐pituitary‐adrenal axis in asthmatic subjects: a dose‐response study. Journal of Allergy & Clinical Immunology 1998 Mar;101(3):312‐9. - PubMed
Agertoft 1997 {published data only}
-
- Agertoft L, Pedersen S. Short‐term knemometry and urine cortisol excretion in children treated with fluticasone propionate and budesonide: a dose response study. European Respiratory Journal 1997;10(7):1507‐12. - PubMed
Baki 1998 {published data only}
-
- Baki A, Karaguzel G. Short‐term effects of budesonide, nedocromil sodium and salmeterol on bronchial hyperresponsiveness in childhood asthma. Acta Paediatrica Japonica 1998;40(3):247‐51. - PubMed
Bel 1991 {published data only}
-
- Bel EH, Timmers MC, Zwinderman AH, Dijkman JH, Sterk PJ. The effect of inhaled corticosteroids on the maximal degree of airway narrowing to methacholine in asthmatic subjects. American Review of Respiratory Disease 1991;143(1):109‐13. - PubMed
Boner 1995 {published and unpublished data}
-
- Boner AL, Comis A, Schiassi M, Venge P, Piacentini GL. Bronchial reactivity in asthmatic children at high and low altitude. Effect of budesonide. American Journal of Respiratory and Critical Care Medicine 1995;151(4):1194‐200. - PubMed
Burke 1996 {published data only}
Busse 1998 {published data only}
-
- Busse WW, Chervinsky P, Condemi J, Lumry WR, Petty TL, Rennard S, et al. Budesonide delivered by Turbuhaler is effective in a dose‐dependent fashion when used in the treatment of adult patients with chronic asthma. Journal of Allergy and Clinical Immunology 1998;101(4 Pt 1):457‐63. - PubMed
Campbell 1991 {published data only}
-
- Campbell LM, Watson DG, Venables TL, Taylor MD, Richardson PDI. Once daily budesonide turbohaler compared to placebo as initial prophylactic therapy for asthma. British Journal of Clinical Research 1991;2:111‐22.
Cockcroft 1995 {published data only}
-
- Cockcroft DW, Swystun VA, Bhagat R. Interaction of inhaled beta 2 agonist and inhaled corticosteroid on airway responsiveness to allergen and methacholine. American Journal of Respiratory and Critical Care Medicine 1995;152(5 Pt 1):1485‐9. - PubMed
de Baets 1990 {published data only}
-
- Baets FM, Goeteyn M, Kerrebijn KF. The effect of two months of treatment with inhaled budesonide on bronchial responsiveness to histamine and house‐dust mite antigen in asthmatic children. American Review of Respiratory Disease 1990;142(3):581‐6. - PubMed
de Jong 1996 {published data only}
-
- Jong JW, Mark TW, Koeter GH, Postma DS. Rebound airway obstruction and responsiveness after cessation of terbutaline: effects of budesonide. American Journal of Respiratory and Critical Care Medicine 1996;153(1):70‐5. - PubMed
Essen‐Zandvliet 1992 {published data only}
-
- Merkus PJ, Essen‐Zandvliet EE, Duiverman EJ, Houwelingen HC, Kerrebijn KF, Quanjer PH. Long‐term effect of inhaled corticosteroids on growth rate in adolescents with asthma. Pediatrics 1993;91(6):1121‐6. - PubMed
-
- Essen‐Zandvliet EE. Long‐term intervention in childhood asthma: the Dutch study results. Dutch Chronic Nonspecific Lung Disease Study Group. Monaldi Archives of Chest Disease 1995;50(3):201‐7. - PubMed
-
- Essen‐Zandvliet EEM, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled corticosteroids and/or beta‐2‐agonists on lung function, airway responsiveness, and symptoms in children with asthma. American Review of Respiratory Disease 1992;146(3):547‐554. - PubMed
Fuller 1991 {published data only}
-
- Fuller RW, Choudry NB, Eriksson G. Action of budesonide on asthmatic bronchial hyperresponsiveness. Effects on directly and indirectly acting bronchoconstrictors. Chest 1991;100(3):670‐4. - PubMed
Gauvreau 1996 {published and unpublished data}
-
- Gauvreau GM, Doctor J, Watson RM, Jordana M, O'Byrne PM. Effects of inhaled budesonide on allergen‐induced airway responses and airway inflammation. American Journal of Respiratory and Critical Care Medicine 1996;154(5):1267‐71. - PubMed
Gleeson 1988 {published data only}
Haahtela 1994 {published data only}
-
- Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. New England Journal of Medicine 1994;331(11):700‐5. - PubMed
Henriksen 1985 {published data only}
Heuck 1997b {published data only}
-
- Heuck C, Wolthers OD, Hansen M, Kollerup G. Short‐term growth and collagen turnover in asthmatic adolescents treated with the inhaled glucocorticoid budesonide. Steroids 1997;62(10):659‐64. - PubMed
Jatakanon 1998 {published data only}
-
- Jatakanon A, Lim S, Chung KF, Barnes PJ. An inhaled steroid improves markers of airway inflammation in patients with mild asthma. European Respiratory Journal 1998 Nov;12(5):1084‐8. - PubMed
Jonasson 1998 {published data only}
-
- Jonasson G, Carlsen KH, Blomqvist P. Clinical efficacy of low‐dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. European Respiratory Journal 1998;12(5):1099‐104. - PubMed
Jones 1994 {published data only}
-
- Jones AH, Langdon CG, Lee PS, Lingham SA, Nankani JP, Follows RM, et al. Pulmicort Turbohaler once daily as initial prophylactic therapy for asthma. Respiratory Medicine 1994;88(4):293‐9. - PubMed
Juniper 1990 {published data only}
-
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long‐term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid‐dependent asthmatics. American Review of Respiratory Disease 1990;142(4):832‐6. - PubMed
Kharitonov 1996 {published data only}
-
- Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. American Journal of Respiratory and Critical Care Medicine 1996;153(1):454‐7. - PubMed
Kivity 1994 {published data only}
-
- Kivity S, Fireman E, Greif J, Schwarz Y, Topilsky M. Effect of budesonide on bronchial hyperresponsiveness and pulmonary function in patients with mild to moderate asthma. Annals of Allergy 1994;72(4):333‐6. - PubMed
Kuo 1994 {published data only}
-
- Kuo HP, Yu TR, Yu CT. Hypodense eosinophil number relates to clinical severity, airway hyperresponsiveness and response to inhaled corticosteroids in asthmatic subjects. European Respiratory Journal 1994;7(8):1452‐9. - PubMed
Lorentzson 1990 {published data only}
Nelson 1998 {published data only}
-
- Nelson HS, Bernstein IL, Fink J, Edwards TB, Spector SL, Storms WW, et al. Oral glucocorticosteroid‐sparing effect of budesonide administered by Turbuhaler: a double‐blind, placebo‐controlled study in adults with moderate‐to‐severe chronic asthma. Pulmicort Turbuhaler Study Group. Chest 1998;113(5):1264‐71. - PubMed
O'Byrne 1996 {published data only}
-
- O'Byrne P, Cuddy L, Taylor DW, Birch S, Morris J, Syrotuik J. Efficacy and cost benefit of inhaled corticosteroids in patients considered to have mild asthma in primary care practice. Canadian Respiratory Journal 1996;3(3):169‐75.
O'Connor 1992 {published data only}
-
- Evans PM, O'Connor BJ, Fuller RW, Barnes PJ, Chung KF. Effect of inhaled corticosteroids on peripheral blood eosinophil counts and density profiles in asthma. Journal of Allergy & Clinical Immunology 1993;91(2):643‐50. - PubMed
-
- O'Connor BJ, Ridge SM, Barnes PJ, Fuller RW. Greater effect of inhaled budesonide on adenosine 5'‐monophosphate‐induced than on sodium‐metabisulfite‐induced bronchoconstriction in asthma. American Review of Respiratory Disease 1992;146(3):560‐4. - PubMed
Osterman 1997 {published data only}
-
- Osterman K, Carlholm M, Ekelund J, Kiviloog J, Nikander K, Nilholm L, et al. Effect of 1 year daily treatment with 400 microg budesonide (Pulmicort Turbuhaler) in newly diagnosed asthmatics. European Respiratory Journal 1997;10(10):2210‐5. - PubMed
Prieto 1994 {published data only}
-
- Prieto L, Berto JM, Gutierrez V, Tornero C. Effect of inhaled budesonide on seasonal changes in sensitivity and maximal response to methacholine in pollen‐sensitive asthmatic subjects. European Respiratory Journal 1994;7(10):1845‐51. - PubMed
Rodriguez 1991 {published data only}
-
- Estrada Rodriguez JL, Belchi Hernandez J, Florido Lopez JF, Lopez Serrano C, Martinez Alzamora F, Ojeda Casas JA. Short‐term treatment of asthma with budesonide versus placebo. Journal of Investigational Allergology & Clinical Immunology 1991;1(4):266‐70. - PubMed
Sekerel 1997a {published data only}
-
- Sekerel BE, Tuncer A, Saraclar Y, Adalioglu G. Inhaled budesonide reduces lung hyperinflation in children with asthma. Acta Paediatrica 1997;86(9):932‐6. - PubMed
Shapiro 1998a {published data only}
-
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH, et al. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. Journal of Pediatrics 1998;132(6):976‐82. - PubMed
Swystun 1998 {published data only}
-
- Swystun VA, Bhagat R, Kalra S, Jennings B, Cockcroft DW. Comparison of 3 different doses of budesonide and placebo on the early asthmatic response to inhaled allergen. Journal of Allergy & Clinical Immunology 1998;102(3):363‐7. - PubMed
Tan 1998 {published data only}
-
- Tan WC, Koh TH, Hay CS, Taylor E. The effect of inhaled budesonide on the diurnal variation in airway mechanics, airway responsiveness and serum neutrophil chemotactic activity in Asian patients with predominant nocturnal asthma. Respirology 1998;3(1):13‐20. - PubMed
Toogood 1990 {published and unpublished data}
-
- Toogood JH, Frankish CW, Jennings BH, Baskerville JC, Borga O, Lefcoe NM, et al. A study of the mechanism of the antiasthmatic action of inhaled budesonide. Journal of Allergy & Clinical Immunology 1990;85(5):872‐80. - PubMed
Vathenen 1991 {published data only}
-
- Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Time course of change in bronchial reactivity with an inhaled corticosteroid in asthma. American Review of Respiratory Disease 1991;143(6):1317‐21. - PubMed
Wempe 1992a {published data only}
-
- Wempe JB, Tammeling EP, Koeter GH, Hakansson L, Venge P, Postma DS. Blood eosinophil numbers and activity during 24 hours: Effects of treatment with budesonide and bambuterol. Journal of Allergy & Clinical Immunology 1992;90(5):757‐65. - PubMed
-
- Wempe JB, Tammeling EP, Postma DS, Auffarth B, Teengs JP, Koeter GH. Effects of budesonide and bambuterol on circadian variation of airway responsiveness and nocturnal symptoms of asthma. Journal of Allergy & Clinical Immunology 1992;90(3 I):349‐57. - PubMed
Wempe 1992b {published data only}
-
- Wempe JB, Tammeling EP, Koeter GH, Hakansson L, Venge P, Postma DS. Blood eosinophil numbers and activity during 24 hours: Effects of treatment with budesonide and bambuterol. Journal of Allergy & Clinical Immunology 1992;90(5):757‐65. - PubMed
-
- Wempe JB, Tammeling EP, Postma DS, Auffarth B, Teengs JP, Koeter GH. Effects of budesonide and bambuterol on circadian variation of airway responsiveness and nocturnal symptoms of asthma. Journal of Allergy & Clinical Immunology 1992;90(3 I):349‐57. - PubMed
Wong 1994 {published data only}
-
- Wong CS, Wahedna I, Pavord ID, Tattersfield AE. Effect of regular terbutaline and budesonide on bronchial reactivity to allergen challenge. American Journal of Respiratory & Critical Care Medicine 1994;150(5 Pt 1):1268‐73. - PubMed
Wongtim 1995 {published data only}
-
- Wongtim S, Mogmued S, Chareonlap P, Limthongkul S. Effect of inhaled corticosteroids on bronchial hyperresponsiveness in patients with mild asthma. Asian Pacific Journal of Allergy & Immunology 1995;13(2):81‐5. - PubMed
Yates 1996 {published data only}
-
- Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long‐acting inhaled beta 2‐agonist [published erratum appears in Am J Respir Crit Care Med 1997 Apr;155(4):1491]. American Journal of Respiratory & Critical Care Medicine 1996;154(6 Pt 1):1603‐7. - PubMed
References to studies excluded from this review
Bisgaard 1990 {published data only}
-
- Bisgaard H, Munck SL, Nielsen JP, Petersen W, Ohlsson SV. Inhaled budesonide for treatment of recurrent wheezing in early childhood. Lancet 1990;336(8716):649‐51. - PubMed
Bisgaard 1993 {published data only}
-
- Bisgaard H. Systemic activity of inhaled topical steroid in toddlers studied by knemometry. Acta Paediatrica 1993;82(12):1066‐71. - PubMed
Booms 1997 {published data only}
-
- Booms P, Cheung D, Timmers MC, Zwinderman AH, Sterk PJ. Protective effect of inhaled budesonide against unlimited airway narrowing to methacholine in atopic patients with asthma. Journal of Allergy & Clinical Immunology 1997;99(3):330‐7. - PubMed
Clark 1997 {published data only}
-
- Clark DJ, Lipworth BJ. Evaluation of corticotropin releasing factor stimulation and basal markers of hypothalamic‐pituitary‐adrenal axis suppression in asthmatic patients. Chest 1997;112(5):1248‐52. - PubMed
Connett 1993 {published data only}
-
- Connett GJ, Lenney W, McConchie SM. The cost effectiveness of budesonide in severe asthmatics aged one to three years. British Journal of Medical Economics 1993;6:127‐34.
Dahl 1982 {published data only}
-
- Dahl R, Johansson SA. Effect on lung function of budesonide by inhalation, terbutaline s.c. and placebo given simultaneously or as single treatments. European Journal of Respiratory Diseases ‐ Supplement 1982;122:132‐7. - PubMed
de Blic 1996 {published data only}
-
- Blic J, Delacourt C, Bourgeois M, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double‐blind study. Journal of Allergy & Clinical Immunology 1996;98(1):14‐20. - PubMed
Engel 1991 {published data only}
-
- Engel T, Dirksen A, Heinig JH, Nielsen NH, Weeke B, Johansson SA. Single‐dose inhaled budesonide in subjects with chronic asthma. Allergy: European Journal of Allergy & Clinical Immunology 1991;46(7):547‐53. - PubMed
Essen Zandvliet 1993 {published data only}
-
- Essen‐Zandvliet EE, Hop WC, Jong H, Ferwerda A, Kerrebijn KF. Minor acute effect of an inhaled corticosteroid (budesonide) on bronchial hyperresponsiveness to methacholine in children with asthma. European Respiratory Journal 1993;6(3):383‐6. - PubMed
Fuglsang 1995 {published data only}
-
- Fuglsang G, Agertoft L, Vikre‐Jorgensen J, Pedersen S. Influence of budesonide on the response to inhaled terbutaline in children with mild asthma. Pediatric Allergy & Immunology 1995;6(2):103‐8. - PubMed
Heuck 1997a {published data only}
-
- Heuck C, Wolthers OD. Calculation of knemometric growth rates in group studies of children treated with exogenous glucocorticoids. Annals of Human Biology 1997;24(5):411‐8. - PubMed
Ilangovan 1993 {published data only}
Kidney 1997 {published data only}
-
- Kidney JC, Boulet LP, Hargreave FE, et al. Evaluation of single‐dose inhaled corticosteroid activity with an allergen challenge model. Journal of Allergy & Clinical Immunology 1997;100(1):65‐70. - PubMed
Kozak‐Szkopek 1997 {published data only}
-
- Kozak‐Szkopek E, Ulmer WT. Inhalative glucocorticosteroids in chronic bronchitis [Inhalative budesonid‐therapie bei chronischer bronchitis]. Atemwegs Und Lungenkrankheiten 1997;23(9):542‐6. [MEDLINE: ; CN‐00230575 ‐ CCTR]
Noble 1992 {published data only}
Paggiaro 1994 {published and unpublished data}
-
- Paggiaro PL, Dente FL, Morelli MC, Bancalari L, Franco A, Giannini D, et al. Postallergen inhaled budesonide reduces late asthmatic response and inhibits the associated increase of airway responsiveness to methacholine in asthmatics. American Journal of Respiratory & Critical Care Medicine 1994;149(6):1447‐51. - PubMed
Rocca 1996 {published data only}
-
- Rocca S, Malka M, Caekert A, Marty M. Influence of inhalation device on asthmatic patients quality of life: Switch MDI to Turbuhaler. Allergie Et Immunologie 1996;28(6):202‐17.
Shapiro 1998b {published data only}
-
- Shapiro G, Mendelson L, Kraemer MJ, Cruz‐Rivera M, Walton‐Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid‐dependent, persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(5):789‐96. - PubMed
Sutochnikova 1996 {published data only}
-
- Sutochnikova OA, Samsonova MV, Cherniak AV, Cherniaev AL, Sokolov AS, Chuchalin AG. [The effect of the Russian inhalant glucocorticosteroid budesonide on bronchial inflammation and hyperreactivity during the long‐term treatment of bronchial asthma patients]. Ter Arkh 1996;68(3):48‐50. - PubMed
Van Bever 1990 {published data only}
-
- Bever HP, Schuddinck L, Wojciechowski M, Stevens WJ. Aerosolized budesonide in asthmatic infants: a double blind study. Pediatric Pulmonology 1990;9(3):177‐80. - PubMed
Waalkens 1990 {published data only}
-
- Waalkens HJ, Essen‐Zandvliet EE, Gerritsen J, Duiverman EJ, Kerrebijn KF, Knol K. The effect of an inhaled corticosteroid (budesonide) on exercise‐induced asthma in children. Dutch CNSLD Study Group. European Respiratory Journal 1993;6(5):652‐6. - PubMed
Wilson 1995 {published data only}
Wilson 1998 {published data only}
-
- Wilson AM, Brewster HJ, Lipworth BJ. Dose‐response comparison of systemic bioactivity with inhaled budesonide and triamcinolone acetonide in asthmatic adults. Journal of Allergy & Clinical Immunology 1998;102(5):751‐6. - PubMed
Wolthers 1998 {published data only}
-
- Wolthers OD, Heuck C. Differential effects of inhaled budesonide on serum osteocalcin in children and adolescents with asthma. Pediatric Allergy & Immunology 1998;9(3):150‐5. - PubMed
References to studies awaiting assessment
Chyrek‐Borowska 1994 {published data only}
-
- Chyrek‐Borowska S, Siergiejko Z, Poludniewska B. [Horacort (new steroid aerosol preparation) for treatment of bronchial asthma and allergic rhinitis]. Pneumonologia i Alergologia Polska 1994;62(1‐2):56‐63. - PubMed
Damsbo 1994 {published data only}
-
- Damsbo N. [Budesonide in the treatment of mild asthma. A placebo‐controlled multicenter study in general practice]. Ugeskr Laeger 1994;156(43):6384‐8. - PubMed
Gorski 1993 {published data only}
-
- Gorski P, Palczynsk C. [Clinical evaluation of budesonide forte action in patients with atopic bronchial asthma]. Polski Tygodnik Lekarski 1993;48:190‐2. - PubMed
Sekerel 1997b {published data only}
-
- Sekerel BE, Tuncer A, Saraclar Y, Adalioglu G. The effect of inhaled budesonide on bronchial hyperreactivity in children with bronchial asthma. Cocuk Sagligi Ve Hastaliklari Dergisi 1997;40(3):351‐360.
Additional references
BTS 1997
-
- British Thoracic Society. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐20.
CONSORT 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. - PubMed
GINA 1995
-
- National Asthma Education and Prevention Program. Global initiative for asthma management and prevention NHBLI/WHO workshop report. Bethseda, MD: National Institute of Health, 1995. [NIH Publication No. 95‐3659]
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996 Feb;17(1):1‐12. - PubMed
NHLBI 1997
-
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue NIH Publication No. 97‐4051.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

