Early postnatal ataxia and abnormal cerebellar development in mice lacking Xeroderma pigmentosum Group A and Cockayne syndrome Group B DNA repair genes
- PMID: 11687625
- PMCID: PMC60879
- DOI: 10.1073/pnas.231329598
Early postnatal ataxia and abnormal cerebellar development in mice lacking Xeroderma pigmentosum Group A and Cockayne syndrome Group B DNA repair genes
Abstract
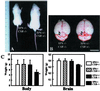
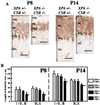
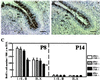
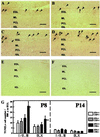
Xeroderma pigmentosum (XP) and Cockayne syndrome (CS) are rare autosomal recessive disorders associated with a defect in the nucleotide excision repair (NER) pathway required for the removal of DNA damage induced by UV light and distorting chemical adducts. Although progressive neurological dysfunction is one of the hallmarks of CS and of some groups of XP patients, the causative mechanisms are largely unknown. Here we show that mice lacking both the XPA (XP-group A) and CSB (CS-group B) genes in contrast to the single mutants display severe growth retardation, ataxia, and motor dysfunction during early postnatal development. Their cerebella are hypoplastic and showed impaired foliation and stunted Purkinje cell dendrites. Reduced neurogenesis and increased apoptotic cell death occur in the cerebellar external granular layer. These findings suggest that XPA and CSB have additive roles in the mouse nervous system and support a crucial role for these genes in normal brain development.
Figures


Comment in
-
DNA repair on the brain.Proc Natl Acad Sci U S A. 2001 Nov 6;98(23):12860-2. doi: 10.1073/pnas.241519498. Proc Natl Acad Sci U S A. 2001. PMID: 11698674 Free PMC article. No abstract available.
Similar articles
-
Mutations in Cockayne Syndrome-Associated Genes (Csa and Csb) Predispose to Cisplatin-Induced Hearing Loss in Mice.J Neurosci. 2016 Apr 27;36(17):4758-70. doi: 10.1523/JNEUROSCI.3890-15.2016. J Neurosci. 2016. PMID: 27122034 Free PMC article.
-
Xeroderma pigmentosum and molecular cloning of DNA repair genes.Anticancer Res. 1996 Mar-Apr;16(2):693-708. Anticancer Res. 1996. PMID: 8687116 Review.
-
Age-related neuronal degeneration: complementary roles of nucleotide excision repair and transcription-coupled repair in preventing neuropathology.PLoS Genet. 2011 Dec;7(12):e1002405. doi: 10.1371/journal.pgen.1002405. Epub 2011 Dec 8. PLoS Genet. 2011. PMID: 22174697 Free PMC article.
-
DNA repair-deficient Xpa and Xpa/p53+/- knock-out mice: nature of the models.Toxicol Pathol. 2001;29 Suppl:109-16. doi: 10.1080/019262301753178519. Toxicol Pathol. 2001. PMID: 11695546 Review.
-
Cockayne syndrome and xeroderma pigmentosum.Neurology. 2000 Nov 28;55(10):1442-9. doi: 10.1212/wnl.55.10.1442. Neurology. 2000. PMID: 11185579 Free PMC article. Review.
Cited by
-
Current Perspective in the Discovery of Anti-aging Agents from Natural Products.Nat Prod Bioprospect. 2017 Oct;7(5):335-404. doi: 10.1007/s13659-017-0135-9. Epub 2017 May 31. Nat Prod Bioprospect. 2017. PMID: 28567542 Free PMC article. Review.
-
Loss of ercc1 results in a time- and dose-dependent reduction of proliferating early hematopoietic progenitors.Anemia. 2012;2012:783068. doi: 10.1155/2012/783068. Epub 2012 Jun 3. Anemia. 2012. PMID: 22701168 Free PMC article.
-
Nucleotide excision repair genes shaping embryonic development.Open Biol. 2019 Oct 31;9(10):190166. doi: 10.1098/rsob.190166. Epub 2019 Oct 30. Open Biol. 2019. PMID: 31662099 Free PMC article. Review.
-
Rescue of premature aging defects in Cockayne syndrome stem cells by CRISPR/Cas9-mediated gene correction.Protein Cell. 2020 Jan;11(1):1-22. doi: 10.1007/s13238-019-0623-2. Epub 2019 Apr 30. Protein Cell. 2020. PMID: 31037510 Free PMC article.
-
Neuroimaging in Cockayne syndrome.AJNR Am J Neuroradiol. 2010 Oct;31(9):1623-30. doi: 10.3174/ajnr.A2135. Epub 2010 Jun 3. AJNR Am J Neuroradiol. 2010. PMID: 20522568 Free PMC article.
References
-
- Bootsma D, Kraemer K H, Cleaver J E, Hoeijmakers J H J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 2001. pp. 677–703.
-
- de Laat W L, Jaspers N G J, Hoeijmakers H J. Genes Dev. 1999;13:768–785. - PubMed
-
- Nance M A, Berry S A. Am J Med Genet. 1992;42:68–82. - PubMed
-
- de Vries A, van Oostrom C T, Hofhuis F M, Dortant P M, Berg R J, de Gruijl F R, Wester P W, van Kreijl C F, Capel P J, van Steeg H, Verbeek S J. Nature (London) 1995;377:169–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

