The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics
- PMID: 11687658
- PMCID: PMC60805
- DOI: 10.1073/pnas.241407898
The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics
Abstract
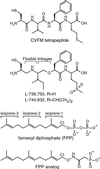
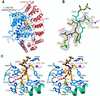
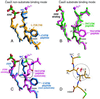
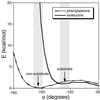
Protein farnesyltransferase (FTase) catalyzes the attachment of a farnesyl lipid group to the cysteine residue located in the C-terminal tetrapeptide of many essential signal transduction proteins, including members of the Ras superfamily. Farnesylation is essential both for normal functioning of these proteins, and for the transforming activity of oncogenic mutants. Consequently FTase is an important target for anti-cancer therapeutics. Several FTase inhibitors are currently undergoing clinical trials for cancer treatment. Here, we present the crystal structure of human FTase, as well as ternary complexes with the TKCVFM hexapeptide substrate, CVFM non-substrate tetrapeptide, and L-739,750 peptidomimetic with either farnesyl diphosphate (FPP), or a nonreactive analogue. These structures reveal the structural mechanism of FTase inhibition. Some CaaX tetrapeptide inhibitors are not farnesylated, and are more effective inhibitors than farnesylated CaaX tetrapeptides. CVFM and L-739,750 are not farnesylated, because these inhibitors bind in a conformation that is distinct from the TKCVFM hexapeptide substrate. This non-substrate binding mode is stabilized by an ion pair between the peptide N terminus and the alpha-phosphate of the FPP substrate. Conformational mapping calculations reveal the basis for the sequence specificity in the third position of the CaaX motif that determines whether a tetrapeptide is a substrate or non-substrate. The presence of beta-branched amino acids in this position prevents formation of the non-substrate conformation; all other aliphatic amino acids in this position are predicted to form the non-substrate conformation, provided their N terminus is available to bind to the FPP alpha-phosphate. These results may facilitate further development of FTase inhibitors.
Figures
Similar articles
-
The basis for K-Ras4B binding specificity to protein farnesyltransferase revealed by 2 A resolution ternary complex structures.Structure. 2000 Feb 15;8(2):209-22. doi: 10.1016/s0969-2126(00)00096-4. Structure. 2000. PMID: 10673434
-
A non-peptide mimetic of Ras-CAAX: selective inhibition of farnesyltransferase and Ras processing.J Biol Chem. 1995 Jan 13;270(2):660-4. doi: 10.1074/jbc.270.2.660. J Biol Chem. 1995. PMID: 7822292
-
Amino acid residues that define both the isoprenoid and CAAX preferences of the Saccharomyces cerevisiae protein farnesyltransferase. Creating the perfect farnesyltransferase.J Biol Chem. 1998 Apr 17;273(16):9472-9. doi: 10.1074/jbc.273.16.9472. J Biol Chem. 1998. PMID: 9545274
-
Advances in the development of farnesyltransferase inhibitors: substrate recognition by protein farnesyltransferase.J Cell Biochem Suppl. 1997;27:12-9. J Cell Biochem Suppl. 1997. PMID: 9591188 Review.
-
Structure based design of benzophenone-based non-thiol farnesyltransferase inhibitors.Curr Pharm Des. 2002;8(19):1713-22. doi: 10.2174/1381612023394061. Curr Pharm Des. 2002. PMID: 12171543 Review.
Cited by
-
Zinc-binding cysteines: diverse functions and structural motifs.Biomolecules. 2014 Apr 17;4(2):419-34. doi: 10.3390/biom4020419. Biomolecules. 2014. PMID: 24970223 Free PMC article. Review.
-
Computational studies of the farnesyltransferase ternary complex part II: the conformational activation of farnesyldiphosphate.Biochemistry. 2007 Oct 30;46(43):12375-81. doi: 10.1021/bi701324t. Epub 2007 Oct 6. Biochemistry. 2007. PMID: 17918965 Free PMC article.
-
Potent, Plasmodium-selective farnesyltransferase inhibitors that arrest the growth of malaria parasites: structure-activity relationships of ethylenediamine-analogue scaffolds and homology model validation.J Med Chem. 2008 Sep 11;51(17):5176-97. doi: 10.1021/jm800113p. Epub 2008 Aug 8. J Med Chem. 2008. PMID: 18686940 Free PMC article.
-
Farnesyl diphosphate analogues with aryl moieties are efficient alternate substrates for protein farnesyltransferase.Biochemistry. 2012 Oct 16;51(41):8307-19. doi: 10.1021/bi3011362. Epub 2012 Oct 2. Biochemistry. 2012. PMID: 22989235 Free PMC article.
-
Expression of functional Plasmodium falciparum enzymes using a wheat germ cell-free system.Eukaryot Cell. 2013 Dec;12(12):1653-63. doi: 10.1128/EC.00222-13. Epub 2013 Oct 11. Eukaryot Cell. 2013. PMID: 24123271 Free PMC article.
References
-
- Casey P J. Science. 1995;268:221–225. - PubMed
-
- Zhang F L, Casey P J. Annu Rev Biochem. 1996;65:241–269. - PubMed
-
- Ashar H R, James L, Gray K, Carr D, Black S, Armstrong L, Bishop W R, Kirschmeier P. J Biol Chem. 2000;275:30451–30457. - PubMed
-
- Schafer W R, Kim R, Sterne R, Thorner J, Kim S-H, Rine J. Science. 1989;245:379–385. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

