Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak
- PMID: 11689432
- PMCID: PMC125712
- DOI: 10.1093/emboj/20.21.5919
Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak
Abstract
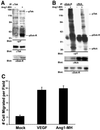
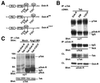
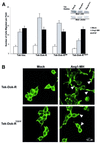
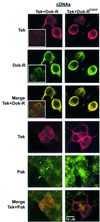
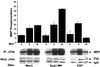
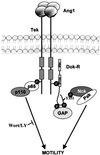
Tek/Tie-2 is an endothelial cell (EC)-specific receptor tyrosine kinase that plays a critical role in angiogenesis via its regulation by the angiopoietin family of growth factor ligands. Angiopoietin-1 (Ang1) can promote EC migration; however, the signaling mechanisms underlying this process remain elusive. Here we demonstrate that Dok-R/Dok-2 can associate with Tek in ECs following Ang1 stimulation, resulting in tyrosine phosphorylation of Dok-R and the subsequent recruitment of Nck and the p21-activating kinase (Pak/Pak1) to the activated receptor. Ang1-mediated migration is increased upon Dok-R overexpression and this requires a functional Nck binding site on Dok-R. Localization of this Dok-R-Nck-Pak complex to the activated Tek receptor at the cellular membrane is coincident with activation of Pak kinase. The ability of Dok-R to bind Nck is required for maximal activation of Pak and overexpression of Pak results in increased Ang1-mediated cell motility. Our study outlines a novel signaling pathway underlying Ang1-driven cell migration that involves Dok-R and its recruitment of Nck and the subsequent activation of Pak.
Figures



References
-
- Adam L., Vadlamudi,R., Kondapaka,S.B., Chernoff,J., Mendelsohn,J. and Kumar,R. (1998) Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem., 273, 28238–28246. - PubMed
-
- Alessi D.R., James,S.R., Downes,C.P., Holmes,A.B., Gaffney,P.R., Reese,C.B. and Cohen,P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol., 7, 261–269. - PubMed
-
- Bokoch G.M., Wang,Y., Bohl,B.P., Sells,M.A., Quilliam,L.A. and Knaus,U.G. (1996) Interaction of the Nck adapter protein with p21-activated kinase (PAK1). J. Biol. Chem., 271, 25746–25749. - PubMed
-
- Carpino N., Wisniewski,D., Strife,A., Marshak,D., Kobayashi,R., Stillman,B. and Clarkson,B. (1997) p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell, 88, 197–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

