Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter
- PMID: 11689434
- PMCID: PMC125690
- DOI: 10.1093/emboj/20.21.5940
Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter
Abstract
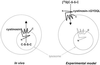
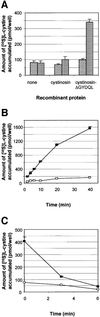
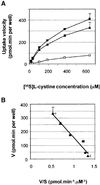
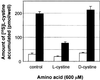
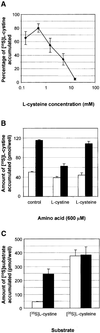
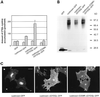
Cystinosis is an inherited lysosomal storage disease characterized by defective transport of cystine out of lysosomes. However, the causative gene, CTNS, encodes a seven transmembrane domain lysosomal protein, cystinosin, unrelated to known transporters. To investigate the molecular function of cystinosin, the protein was redirected from lysosomes to the plasma membrane by deletion of its C-terminal GYDQL sorting motif (cystinosin-DeltaGYDQL), thereby exposing the intralysosomal side of cystinosin to the extracellular medium. COS cells expressing cystinosin-DeltaGYDQL selectively take up L-cystine from the extracellular medium at acidic pH. Disruption of the transmembrane pH gradient or incubation of the cells at neutral pH strongly inhibits the uptake. Cystinosin-DeltaGYDQL is directly involved in the observed cystine transport, since this activity is highly reduced when the GYDQL motif is restored and is abolished upon introduction of a point mutation inducing early-onset cystinosis. We conclude that cystinosin represents a novel H(+)-driven transporter that is responsible for cystine export from lysosomes, and propose that cystinosin homologues, such as mammalian SL15/Lec35 and Saccharomyces cerevisiae ERS1, may perform similar transport processes at other cellular membranes.
Figures



References
-
- Anikster Y., Shotelersuk,V. and Gahl,W.A. (1999) CTNS mutations in patients with cystinosis. Hum. Mutat., 14, 454–458. - PubMed
-
- Attard M., Jean,G., Forestier,L., Cherqui,S., van’t Hoff,W., Broyer,M., Antignac,C. and Town,M. (1999) Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum. Mol. Genet., 8, 2507–2514. - PubMed
-
- Chairoungdua A. et al. (1999) Identification of an amino acid transporter associated with the cystinuria-related type II membrane glycoprotein. J. Biol. Chem., 274, 28845–28848. - PubMed
-
- Cherqui S., Kalatzis,V., Trugnan,G. and Antignac,C. (2001) The targeting of cystinosin to the lysosomal membrane requires a tyrosine-based signal and a novel sorting motif. J. Biol. Chem., 276, 13314–13321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

