Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis
- PMID: 11689440
- PMCID: PMC125709
- DOI: 10.1093/emboj/20.21.5999
Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis
Abstract
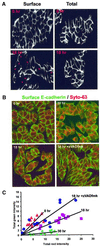
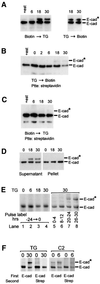
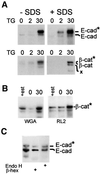
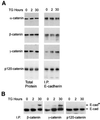
Cellular adhesion is regulated by members of the cadherin family of adhesion receptors and their cytoplasmic adaptor proteins, the catenins. Adhesion complexes are regulated by recycling from the plasma membrane and proteolysis during apoptosis. We report that in MCF-7, MDA-MB-468 and MDCK cells, induction of apoptosis by agents that cause endoplasmic reticulum (ER) stress results in O-glycosylation of both beta-catenin and the E-cadherin cytoplasmic domain. O-glycosylation of newly synthesized E-cadherin blocks cell surface transport, resulting in reduced intercellular adhesion. O-glycosylated E-cadherin still binds to beta- and gamma-catenin, but not to p120-catenin. Although O-glycosylation can be inhibited with caspase inhibitors, cleavage of caspases associated with the ER or Golgi complex does not correlate with E-cadherin O-glycosylation. However, agents that induce apoptosis via mitochondria do not lead to E-cadherin O-glycosylation, and decrease adhesion more slowly. In MCF-7 cells, this is due to degradation of E-cadherin concomitant with cleavage of caspase-7 and its substrate poly(ADP-ribose) polymerase. We conclude that cytoplasmic O-glycosylation is a novel, rapid mechanism for regulating cell surface transport exploited to down-regulate adhesion in some but not all apoptosis pathways.
Figures

References
-
- Anastasiadis P.Z., Moon,S.Y., Thoresen,M.A., Mariner,D.J., Crawford,H.C., Zheng,Y. and Reynolds,A.B. (2000) Inhibition of RhoA by p120 catenin. Nature Cell Biol., 2, 637–644. - PubMed
-
- Annis M., Zamzami,N., Zhu,W., Penn,L.Z., Kroemer,G., Leber,B. and Andrews,D.W. (2000) Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene, 20, 1939–1952. - PubMed
-
- Comer F.I. and Hart,G.W. (2000) O-Glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J. Biol. Chem., 275, 29179–29182. - PubMed
-
- Daniel J.M. and Reynolds,A.B. (1997) Tyrosine phosphorylation and cadherin/catenin function. Bioessays, 19, 883–891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

