Two mechanisms activate PTPalpha during mitosis
- PMID: 11689444
- PMCID: PMC125689
- DOI: 10.1093/emboj/20.21.6037
Two mechanisms activate PTPalpha during mitosis
Abstract
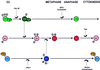
We show that, dependent on serine hyperphosphorylation, protein tyrosine phosphatase alpha (PTPalpha) is activated by two different mechanisms during mitosis: its specific activity increases and its inhibitory binding to Grb2 decreases. The latter effect probably abates Grb2 inhibition of the phosphotyrosine displacement process that is required specifically for Src dephosphorylation and causes a mitotic increase in transient PTPalpha-Src binding. Thus, part of the increased protein tyrosine phosphatase activity may be specific for Src family members. These effects cease along with Src activation when cells exit mitosis. Src is not activated in mitosis in PTPalpha-knockout cells, indicating a unique mitotic role for this phosphatase. The activation of PTPalpha, combined with the effects of mitotic Cdc2-mediated phosphorylations of Src, quantitatively accounts for the mitotic activation of Src, indicating that PTPalpha is the membrane-bound, serine phosphorylation-activated, protein tyrosine phosphatase that activates Src during mitosis.
Figures



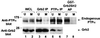




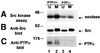
Similar articles
-
Mitotic activation of protein-tyrosine phosphatase alpha and regulation of its Src-mediated transforming activity by its sites of protein kinase C phosphorylation.J Biol Chem. 2002 Jun 14;277(24):21922-9. doi: 10.1074/jbc.M201394200. Epub 2002 Mar 28. J Biol Chem. 2002. PMID: 11923305 Free PMC article.
-
A phosphotyrosine displacement mechanism for activation of Src by PTPalpha.EMBO J. 2000 Mar 1;19(5):964-78. doi: 10.1093/emboj/19.5.964. EMBO J. 2000. PMID: 10698938 Free PMC article.
-
[Changes of phenotypes associated with activated Src by overexpressed PTPalpha in NIH3T3 cells].Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2002 Mar;34(2):193-8. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2002. PMID: 12006995 Chinese.
-
Protein tyrosine phosphatase alpha (PTPalpha): a Src family kinase activator and mediator of multiple biological effects.Curr Top Med Chem. 2003;3(7):821-35. doi: 10.2174/1568026033452320. Curr Top Med Chem. 2003. PMID: 12678847 Review.
-
Src kinase regulation by phosphorylation and dephosphorylation.Biochem Biophys Res Commun. 2005 May 27;331(1):1-14. doi: 10.1016/j.bbrc.2005.03.012. Biochem Biophys Res Commun. 2005. PMID: 15845350 Review.
Cited by
-
Cytokinesis Failure Leading to Chromosome Instability in v-Src-Induced Oncogenesis.Int J Mol Sci. 2017 Apr 12;18(4):811. doi: 10.3390/ijms18040811. Int J Mol Sci. 2017. PMID: 28417908 Free PMC article. Review.
-
c-Src but not Fyn promotes proper spindle orientation in early prometaphase.J Biol Chem. 2012 Jul 20;287(30):24905-15. doi: 10.1074/jbc.M112.341578. Epub 2012 Jun 11. J Biol Chem. 2012. PMID: 22689581 Free PMC article.
-
Apoptosis of estrogen-receptor negative breast cancer and colon cancer cell lines by PTP alpha and src RNAi.Int J Cancer. 2008 May 1;122(9):1999-2007. doi: 10.1002/ijc.23321. Int J Cancer. 2008. PMID: 18183590 Free PMC article.
-
Extracellular domain dependence of PTPalpha transforming activity.Genes Cells. 2010 Jun;15(7):711-724. doi: 10.1111/j.1365-2443.2010.01410.x. Epub 2010 Jun 7. Genes Cells. 2010. PMID: 20545765 Free PMC article.
-
Association of tyrosine phosphatase epsilon with microtubules inhibits phosphatase activity and is regulated by the epidermal growth factor receptor.Mol Cell Biol. 2007 Oct;27(20):7102-12. doi: 10.1128/MCB.02096-06. Epub 2007 Aug 20. Mol Cell Biol. 2007. PMID: 17709387 Free PMC article.
References
-
- Arnott C.H., Sale,E.M., Miller,J. and Sale,G.J. (1999) Use of an antisense strategy to dissect the signaling role of protein-tyrosine phosphatase α. J. Biol. Chem., 274, 26105–26112. - PubMed
-
- Bagrodia S.,I. Chackalaparampil,T.E. Kmiecik and D. Shalloway (1991) Altered tyrosine 527 phosphorylation and mitotic activation of p60c–src. Nature, 349, 172–175. - PubMed
-
- Bagrodia S., Laudano,A.P. and Shalloway,D. (1994) Accessibility of the c-src SH2-domain for binding is increased during mitosis. J. Biol. Chem., 269, 10247–10251. - PubMed
-
- Bhandari B., Lim,K.L. and Pallen,C.J. (1998) Physical and functional interactions between receptor-like protein-tyrosine phosphatase α and p59fyn. J. Biol. Chem., 173, 8691–8698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

