Cross-reactivity between hepatitis C virus and Influenza A virus determinant-specific cytotoxic T cells
- PMID: 11689620
- PMCID: PMC114725
- DOI: 10.1128/JVI.75.23.11392-11400.2001
Cross-reactivity between hepatitis C virus and Influenza A virus determinant-specific cytotoxic T cells
Abstract
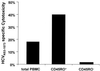
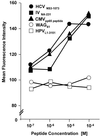
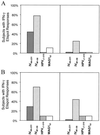
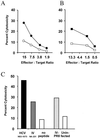
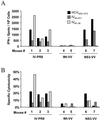
The cellular immune response contributes to viral clearance as well as to liver injury in acute and chronic hepatitis C virus (HCV) infection. An immunodominant determinant frequently recognized by liver-infiltrating and circulating CD8(+) T cells of HCV-infected patients is the HCV(NS3-1073) peptide CVNGVCWTV. Using a sensitive in vitro technique with HCV peptides and multiple cytokines, we were able to expand cytotoxic T cells specific for this determinant not only from the blood of 11 of 20 HCV-infected patients (55%) but also from the blood of 9 of 15 HCV-negative blood donors (60%), while a second HCV NS3 determinant was recognized only by HCV-infected patients and not by seronegative controls. The T-cell response of these healthy blood donors was mediated by memory T cells, which cross-reacted with a novel T-cell determinant of the A/PR/8/34 influenza A virus (IV) that is endogenously processed from the neuraminidase (NA) protein. Both the HCV NS3 and the IV NA peptide displayed a high degree of sequence homology, bound to the HLA-A2 molecule with high affinity, and were recognized by cytotoxic T lymphocytes with similar affinity (10(-8) M). Using the HLA-A2-transgenic mouse model, we then demonstrated directly that HCV-specific T cells could be induced in vivo by IV infection. Splenocytes harvested from IV-infected mice at the peak of the primary response (day 7 effector cells) or following complete recovery (day 21 memory cells) recognized the HCV NS3 peptide, lysed peptide-pulsed target cells, and produced gamma interferon. These results exemplify that host responses to an infectious agent are influenced by cross-reactive memory cells induced by past exposure to heterologous viruses, which could have important consequences for vaccine development.
Figures


Comment in
-
Viral crosstalk: who gets to say what first?Hepatology. 2002 Jun;35(6):1540-3. doi: 10.1002/hep.510350632. Hepatology. 2002. PMID: 12029643 No abstract available.
References
-
- Advisory Committee on Immunization Practices. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1999;48:1–28. - PubMed
-
- Anderson R W, Bennink J R, Yewdell J W, Maloy W L, Coligan J E. Influenza basic polymerase 2 peptides are recognized by influenza nucleoprotein-specific cytotoxic T lymphocytes. Mol Immunol. 1992;29:1089–1096. - PubMed
-
- Bernard H U, Chan S Y, Manos M M, Ong C K, Villa L L, Delius H, Peyton C L, Bauer H M, Wheeler C M. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994;170:1077–1085. - PubMed
-
- Blok J, Air G M. Comparative nucleotide sequences at the 3′ end of the neuraminidase gene from eleven influenza type A viruses. Virology. 1980;107:50–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

