Palmitoylation of the Rous sarcoma virus transmembrane glycoprotein is required for protein stability and virus infectivity
- PMID: 11689636
- PMCID: PMC114741
- DOI: 10.1128/JVI.75.23.11544-11554.2001
Palmitoylation of the Rous sarcoma virus transmembrane glycoprotein is required for protein stability and virus infectivity
Abstract
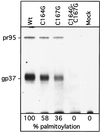
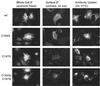
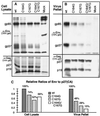
The Rous sarcoma virus (RSV) transmembrane (TM) glycoprotein is modified by the addition of palmitic acid. To identify whether conserved cysteines within the hydrophobic anchor region are the site(s) of palmitoylation, and to determine the role of acylation in glycoprotein function, cysteines at residues 164 and 167 of the TM protein were mutated to glycine (C164G, C167G, and C164G/C167G). In CV-1 cells, palmitate was added to env gene products containing single mutations but was absent in the double-mutant Env. Although mutant Pr95 Env precursors were synthesized with wild-type kinetics, the phenotypes of the mutants differed markedly. Env-C164G had properties similar to those of the wild type, while Env-C167G was degraded faster, and Env containing the double mutant C164G/C167G was very rapidly degraded. Degradation occurred after transient plasma membrane expression. The decrease in steady-state surface expression and increased rate of internalization into endosomes and lysosomes paralleled the decrease in palmitoylation observed for the mutants. The phenotypes of mutant viruses were assessed in avian cells in the context of the pATV8R proviral genome. Virus containing the C164G mutation replicated with wild-type kinetics but exhibited reduced peak reverse transcriptase levels. In contrast, viruses containing either the C167G or the C164G/C167G mutation were poorly infectious or noninfectious, respectively. These phenotypes correlated with different degrees of glycoprotein incorporation into virions. Infectious revertants of the double mutant demonstrated the importance of cysteine-167 for efficient plasma membrane expression and Env incorporation. The observation that both cysteines within the membrane-spanning domain are accessible for acylation has implications for the topology of this region, and a model is proposed.
Figures





References
-
- Adams G A, Rose J K. Structural requirements of a membrane-spanning domain for protein anchoring and cell surface transport. Cell. 1985;41:1007–1015. - PubMed
-
- Alvarez E, Girones N, Davis R J. Inhibition of the receptor-mediated endocytosis of diferric transferrin is associated with the covalent modification of the transferrin receptor with palmitic acid. J Biol Chem. 1990;265:16644–16655. - PubMed
-
- Bonner W M, Laskey R A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974;46:83–88. - PubMed
-
- Bosch J V, Schwarz R T. Processing of gPr92env, the precursor to the glycoproteins of Rous sarcoma virus: use of inhibitors of oligosaccharide trimming and glycoprotein transport. Virology. 1984;132:95–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

