A short N-proximal region in the large envelope protein harbors a determinant that contributes to the species specificity of human hepatitis B virus
- PMID: 11689638
- PMCID: PMC114743
- DOI: 10.1128/JVI.75.23.11565-11572.2001
A short N-proximal region in the large envelope protein harbors a determinant that contributes to the species specificity of human hepatitis B virus
Abstract
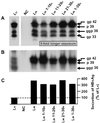
Infection by hepatitis B virus (HBV) is mainly restricted to humans. This species specificity is likely determined at the early phase of the viral life cycle. Since the envelope proteins are the first viral factors to interact with the cell, they represent attractive candidates for controlling the HBV host range. To investigate this assumption, we took advantage of the recent discovery of a second virus belonging to the primate Orthohepadnavirus genus, the woolly monkey HBV (WMHBV). A recombinant plasmid was constructed for the expression of all WMHBV envelope proteins. In additional constructs, N-terminal sequences of the WMHBV large envelope protein were substituted for their homologous HBV counterparts. All wild-type and chimeric WMHBV surface proteins were properly synthesized by transfected human hepatoma cells, and they were competent to replace the original HBV proteins for the production of complete viral particles. The resulting pseudotyped virions were evaluated for their infectious capacity on human hepatocytes in primary culture. Virions pseudotyped with wild-type WMHBV envelope proteins showed a significant loss of infectivity. By contrast, infectivity was completely restored when the first 30 residues of the large protein originated from HBV. Analysis of smaller substitutions within this domain limited the most important region to a stretch of only nine amino acids. Reciprocally, replacement of this motif by WMHBV residues in the context of the HBV L protein significantly reduced infectivity of HBV. Hence this short region of the L protein contributes to the host range of HBV.
Figures
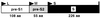


References
-
- Aden D P, Fogel A, Plotkin S, Damjanov I, Knowles B B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature (London) 1979;282:615–616. - PubMed
-
- Bruss V, Hagelsten J, Gerhardt E, Galle P R. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology. 1996;218:396–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

