Porcine encephalomyocarditis virus persists in pig myocardium and infects human myocardial cells
- PMID: 11689644
- PMCID: PMC114749
- DOI: 10.1128/JVI.75.23.11621-11629.2001
Porcine encephalomyocarditis virus persists in pig myocardium and infects human myocardial cells
Abstract
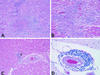
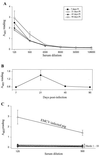
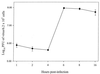
Recent advances toward using pig tissues in human transplantation have made it necessary to determine the risk of transmitting zoonotic viruses from pigs to humans or vice versa. We investigated the suitability of the porcine encephalomyocarditis virus (EMCV) model for such studies by determining its ability to persist in pigs, escape detection by routine serological methods, and infect human cells. Intraperitoneal inoculation of 5-week-old pigs with EMCV-30, a strain isolated from commercial pigs, resulted in acute cellular degeneration, infiltration of lymphocytes, and apoptosis in myocardium in 13 of 15 (86.7%) pigs during the acute phase of disease (3 to 21 days postinfection), followed by less-severe lymphocytic infiltration and apoptosis in 5 of 10 (50%) pigs during the chronic phase of the disease (day 45 to 90 postinfection). In the brain, lymphocytic infiltration, neuronal degeneration, and gliosis were observed in 26 to 33% of pigs in the acute phase of disease whereas perivascular cuffing was the predominant feature during chronic disease. EMCV antigens and RNA were demonstrated in the myocardium and brain during the chronic phase of disease. Analysis of 100 commercial pigs that were negative for EMCV antibodies identified two pig hearts positive for EMCV RNA. Porcine EMCV productively infected primary human cardiomyocytes as demonstrated by immunostaining using a monoclonal antibody specific for EMCV RNA polymerase, which is expressed only in productively infected cells, and by a one-step growth curve that showed production of 100 to 1,000 PFU of virus per cell within 6 h. The findings that porcine EMCV can persist in pig myocardium and can infect human myocardial cells make it an important infectious agent to screen for in pig-to-human cardiac transplants and a good model for xenozoonosis.
Figures





Similar articles
-
Transmission of porcine encephalomyocarditis virus (EMCV) to mice by transplanting EMCV-infected pig tissues.Xenotransplantation. 2003 Nov;10(6):569-76. doi: 10.1034/j.1399-3089.2003.00058.x. Xenotransplantation. 2003. PMID: 14708524
-
A wild-type porcine encephalomyocarditis virus containing a short poly(C) tract is pathogenic to mice, pigs, and cynomolgus macaques.J Virol. 2003 Sep;77(17):9136-46. doi: 10.1128/jvi.77.17.9136-9146.2003. J Virol. 2003. PMID: 12915530 Free PMC article.
-
Transplanting encephalomyocarditis virus-infected porcine islet cells reverses diabetes in recipient mice but also transmits the virus.Xenotransplantation. 2004 Mar;11(2):160-70. doi: 10.1046/j.1399-3089.2003.00102.x. Xenotransplantation. 2004. PMID: 14962278
-
The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--chapter 5: Strategies to prevent transmission of porcine endogenous retroviruses.Xenotransplantation. 2009 Jul-Aug;16(4):239-48. doi: 10.1111/j.1399-3089.2009.00544.x. Xenotransplantation. 2009. PMID: 19799764
-
The encephalomyocarditis virus.Virulence. 2012 Jul 1;3(4):351-67. doi: 10.4161/viru.20573. Epub 2012 Jun 22. Virulence. 2012. PMID: 22722247 Free PMC article. Review.
Cited by
-
Identification and Genomic Characterization of Bovine Boosepivirus A in the United States and Canada.Viruses. 2024 Feb 17;16(2):307. doi: 10.3390/v16020307. Viruses. 2024. PMID: 38400082 Free PMC article.
-
The Origin, Dynamic Morphology, and PI4P-Independent Formation of Encephalomyocarditis Virus Replication Organelles.mBio. 2018 Apr 17;9(2):e00420-18. doi: 10.1128/mBio.00420-18. mBio. 2018. PMID: 29666283 Free PMC article.
-
Adaptive Immune Responses following Senecavirus A Infection in Pigs.J Virol. 2018 Jan 17;92(3):e01717-17. doi: 10.1128/JVI.01717-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29142122 Free PMC article.
-
Dos and don'ts in large animal models of aortic insufficiency.Front Vet Sci. 2022 Sep 2;9:949410. doi: 10.3389/fvets.2022.949410. eCollection 2022. Front Vet Sci. 2022. PMID: 36118338 Free PMC article.
-
Transcriptional profiling of host cell responses to encephalomyocarditis virus (EMCV).Virol J. 2017 Mar 4;14(1):45. doi: 10.1186/s12985-017-0718-4. Virol J. 2017. PMID: 28259172 Free PMC article.
References
-
- Acker M A. Mechanical circulatory support for patients with acute-fulminant myocarditis. Ann Thorac Surg. 2001;71:S73–S76. - PubMed
-
- Billinis C, Paschaleri-Papadopoulou E, Psychas V, Vlemmas J, Leontides S, Koumbati M, Kyriakis S C, Papadopoulos O. Persistence of encephalomyocarditis virus (EMCV) infection in piglets. Vet Microbiol. 1999;70:171–177. - PubMed
-
- Blanchard J L, Soike K F, Baskin G B. Encephalomyocarditis virus infection in African green and squirrel monkeys: comparison of pathological effects. Lab Anim Sci. 1987;37:635–639. - PubMed
-
- Cerutis D R, Bruner R H, Thomas D C, Giron D J. Tropism and histopathology of the D, B, K, and MM variants of encephalomyocarditis virus. J Med Virol. 1989;29:63–69. - PubMed
-
- Craighead J E, Peralata P H, Murnane T G, Shelokov A. Oral infection of swine with encephalomyocarditis virus. J Infect Dis. 1963;112:205–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

