Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains
- PMID: 11689665
- PMCID: PMC114770
- DOI: 10.1128/JVI.75.23.11834-11850.2001
Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains
Abstract
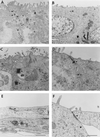
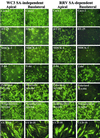
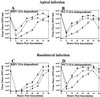
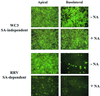
Infection of epithelial cells by some animal rotaviruses, but not human or most animal rotaviruses, requires the presence of N-acetylneuraminic (sialic) acid (SA) on the cell surface for efficient infectivity. To further understand how rotaviruses enter susceptible cells, six different polarized epithelial cell lines, grown on permeable filter membrane supports containing 0.4-microm pores, were infected apically or basolaterally with SA-independent or SA-dependent rotaviruses. SA-independent rotaviruses applied apically or basolaterally were capable of efficiently infecting both sides of the epithelium of all six polarized cell lines tested, while SA-dependent rotaviruses only infected efficiently through the apical surface of five of the polarized cell lines tested. Regardless of the route of virus entry, SA-dependent and SA-independent rotaviruses were released almost exclusively from the apical domain of the plasma membrane of polarized cells before monolayer disruption or cell lysis. The transepithelial electrical resistance (TER) of cells decreased at the same time, irrespective of whether infection with SA-independent rotaviruses occurred apically or basolaterally. The TER of cells infected apically with SA-dependent rotaviruses decreased earlier than that of cells infected basolaterally. Rotavirus infection decreased TER before the appearance of cytopathic effect and cell death and resulted in an increase in the paracellular permeability to [(3)H]inulin as a function of loss of TER. The presence of SA residues on either the apical or basolateral side was determined using a Texas Red-conjugated lectin, wheat germ agglutinin (WGA), which binds SA residues. WGA bound exclusively to SA residues on the apical surface of the cells, confirming the requirement for SA residues on the apical cell membrane for efficient infectivity of SA-dependent rotaviruses. These results indicate that the rotavirus SA-independent cellular receptor is present on both sides of the epithelium, but SA-dependent and SA-independent rotavirus strains infect polarized epithelial cells by different mechanisms, which may be relevant for pathogenesis and selection of vaccine strains. Finally, rotavirus-induced alterations of the epithelial barrier and paracellular permeability suggest that common mechanisms of pathogenesis may exist between viral and bacterial pathogens of the intestinal tract.
Figures



References
-
- Ball J M, Tian P, Zeng C Q-Y, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. - PubMed
-
- Casola A, Estes M K, Crawford S E, Ogra P L, Ernst P B, Garofalo R, Crowe S E. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology. 1998;114:947–955. - PubMed
-
- Ciarlet M, Estes M K. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity. J Gen Virol. 1999;80:943–948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

