The Hsp70-Ydj1 molecular chaperone represses the activity of the heme activator protein Hap1 in the absence of heme
- PMID: 11689685
- PMCID: PMC99961
- DOI: 10.1128/MCB.21.23.7923-7932.2001
The Hsp70-Ydj1 molecular chaperone represses the activity of the heme activator protein Hap1 in the absence of heme
Abstract
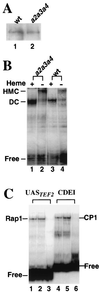
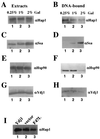
In Saccharomyces cerevisiae, heme directly mediates the effects of oxygen on transcription through the heme activator protein Hap1. In the absence of heme, Hap1 is bound by at least four cellular proteins, including Hsp90 and Ydj1, forming a higher-order complex, termed HMC, and its activity is repressed. Here we purified the HMC and showed by mass spectrometry that two previously unidentified major components of the HMC are the Ssa-type Hsp70 molecular chaperone and Sro9 proteins. In vivo functional analysis, combined with biochemical analysis, strongly suggests that Ssa proteins are critical for Hap1 repression in the absence of heme. Ssa may repress the activities of both Hap1 DNA-binding and activation domains. The Ssa cochaperones Ydj1 and Sro9 appear to assist Ssa in Hap1 repression, and only Ydj1 residues 1 to 172 containing the J domain are required for Hap1 repression. Our results suggest that Ssa-Ydj1 and Sro9 act together to mediate Hap1 repression in the absence of heme and that molecular chaperones promote heme regulation of Hap1 by a mechanism distinct from the mechanism of steroid signaling.
Figures
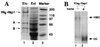
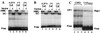
Similar articles
-
A novel mode of chaperone action: heme activation of Hap1 by enhanced association of Hsp90 with the repressed Hsp70-Hap1 complex.J Biol Chem. 2004 Jun 25;279(26):27607-12. doi: 10.1074/jbc.M402777200. Epub 2004 Apr 21. J Biol Chem. 2004. PMID: 15102838
-
The heme activator protein Hap1 represses transcription by a heme-independent mechanism in Saccharomyces cerevisiae.Genetics. 2005 Mar;169(3):1343-52. doi: 10.1534/genetics.104.037143. Epub 2005 Jan 16. Genetics. 2005. PMID: 15654089 Free PMC article.
-
The yeast heme-responsive transcriptional activator Hap1 is a preexisting dimer in the absence of heme.J Biol Chem. 1999 Aug 6;274(32):22770-4. doi: 10.1074/jbc.274.32.22770. J Biol Chem. 1999. PMID: 10428861
-
A unique mechanism of chaperone action: heme regulation of Hap1 activity involves separate control of repression and activation.Protein Pept Lett. 2009;16(6):642-9. doi: 10.2174/092986609788490113. Protein Pept Lett. 2009. PMID: 19519523 Review.
-
Molecular mechanism of heme signaling in yeast: the transcriptional activator Hap1 serves as the key mediator.Cell Mol Life Sci. 1999 Oct 30;56(5-6):415-26. doi: 10.1007/s000180050442. Cell Mol Life Sci. 1999. PMID: 11212295 Free PMC article. Review.
Cited by
-
Farnesylation of Ydj1 is required for in vivo interaction with Hsp90 client proteins.Mol Biol Cell. 2008 Dec;19(12):5249-58. doi: 10.1091/mbc.e08-04-0435. Epub 2008 Oct 1. Mol Biol Cell. 2008. PMID: 18829866 Free PMC article.
-
Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor.Mol Cell Biol. 2007 Nov;27(21):7414-24. doi: 10.1128/MCB.00887-07. Epub 2007 Sep 4. Mol Cell Biol. 2007. PMID: 17785431 Free PMC article.
-
Heme, A Metabolic Sensor, Directly Regulates the Activity of the KDM4 Histone Demethylase Family and Their Interactions with Partner Proteins.Cells. 2020 Mar 22;9(3):773. doi: 10.3390/cells9030773. Cells. 2020. PMID: 32235736 Free PMC article.
-
Influence of Hsp70s and their regulators on yeast prion propagation.Prion. 2009 Apr-Jun;3(2):65-73. doi: 10.4161/pri.3.2.9134. Epub 2009 Apr 29. Prion. 2009. PMID: 19556854 Free PMC article. Review.
-
Regulation of the HAP1 gene involves positive actions of histone deacetylases.Biochem Biophys Res Commun. 2007 Oct 12;362(1):120-125. doi: 10.1016/j.bbrc.2007.07.156. Epub 2007 Aug 8. Biochem Biophys Res Commun. 2007. PMID: 17706600 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
