Signaling through a novel domain of gp130 mediates cell proliferation and activation of Hck and Erk kinases
- PMID: 11689697
- PMCID: PMC99973
- DOI: 10.1128/MCB.21.23.8068-8081.2001
Signaling through a novel domain of gp130 mediates cell proliferation and activation of Hck and Erk kinases
Abstract
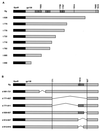
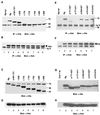
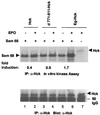
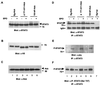
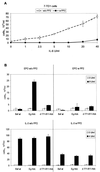
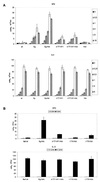
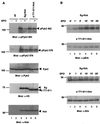
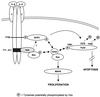
Interleukin-6 (IL-6) induces the activation of the Src family kinase Hck, which is associated with the IL-6 receptor beta-chain, gp130. Here we describe the identification of an "acidic" domain comprising amino acids 771 to 811 of gp130 as a binding region for Hck, which mediates proliferative signaling. The deletion of this region of gp130 (i.e., in deletion mutant d771-811) resulted in a significant reduction of Hck kinase activity and cell proliferation upon stimulation of gp130 compared to wild-type gp130. In addition, d771-811 disrupted the growth factor-stimulated activation of Erk and the dephosphorylation of Pyk2. Based on these findings, we propose a novel, acidic domain of gp130, which is responsible for the activation of Hck, Erk, and Pyk2 and signals cell proliferation upon growth factor stimulation.
Figures
Similar articles
-
TNF-alpha induces tyrosine phosphorylation and recruitment of the Src homology protein-tyrosine phosphatase 2 to the gp130 signal-transducing subunit of the IL-6 receptor complex.J Immunol. 2003 Jul 1;171(1):257-66. doi: 10.4049/jimmunol.171.1.257. J Immunol. 2003. PMID: 12817006
-
SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130.J Biol Chem. 2003 Jan 3;278(1):661-71. doi: 10.1074/jbc.M210552200. Epub 2002 Oct 27. J Biol Chem. 2003. PMID: 12403768
-
Differential inhibition of IL-6-type cytokine-induced STAT activation by PMA.FEBS Lett. 2000 Jul 28;478(1-2):100-4. doi: 10.1016/s0014-5793(00)01826-3. FEBS Lett. 2000. PMID: 10922477
-
Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi's sarcoma-associated herpes virus-encoded viral interleukin 6.Clin Cancer Res. 2000 Mar;6(3):1180-9. Clin Cancer Res. 2000. PMID: 10741750
-
Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway.Biochem J. 1998 Sep 1;334 ( Pt 2)(Pt 2):297-314. doi: 10.1042/bj3340297. Biochem J. 1998. PMID: 9716487 Free PMC article. Review.
Cited by
-
Molecular analysis of signaling events mediated by the cytoplasmic domain of leukemia inhibitory factor receptor alpha subunit.Mol Cell Biochem. 2004 Mar;258(1-2):15-23. doi: 10.1023/b:mcbi.0000012829.10405.e1. Mol Cell Biochem. 2004. PMID: 15030166
-
IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages.J Immunol. 2014 Jan 1;192(1):349-57. doi: 10.4049/jimmunol.1301906. Epub 2013 Nov 27. J Immunol. 2014. PMID: 24285838 Free PMC article.
-
Inhibition of Hematopoietic Cell Kinase Activity Suppresses Myeloid Cell-Mediated Colon Cancer Progression.Cancer Cell. 2017 Apr 10;31(4):563-575.e5. doi: 10.1016/j.ccell.2017.03.006. Cancer Cell. 2017. PMID: 28399411 Free PMC article.
-
A Role for Soluble IL-6 Receptor in Osteoarthritis.J Funct Morphol Kinesiol. 2017;2(3):27. doi: 10.3390/jfmk2030027. Epub 2017 Aug 2. J Funct Morphol Kinesiol. 2017. PMID: 29276788 Free PMC article.
-
IL6-receptor antibody tocilizumab as salvage therapy in severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a retrospective analysis.Ann Hematol. 2020 Apr;99(4):847-853. doi: 10.1007/s00277-020-03968-w. Epub 2020 Feb 21. Ann Hematol. 2020. PMID: 32086584 Free PMC article.
References
-
- Abram C L, Courtneidge S A. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. - PubMed
-
- Adachi T, Pazdrak K, Stafford S, Alam R. The mapping of the Lyn kinase binding site of the common beta subunit of IL-3/granulocyte-macrophage colony-stimulating factor/IL-5 receptor. J Immunol. 1999;162:1496–1501. - PubMed
-
- Anderson S M, Jorgensen B. Activation of Src-related tyrosine kinases by IL-3. J Immunol. 1995;155:1660–1670. - PubMed
-
- Aoki Y, Kim Y T, Stillwell R, Kim T J, Pillai S. The SH2 domains of Src family kinases associate with Syk. J Biol Chem. 1995;270:15658–15663. - PubMed
-
- Avraham H, Park S Y, Schinkmann K, Avraham S. Raftk/Pyk2-mediated cellular signaling. Cell Signal. 2000;12:123–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
