Glycosylation defects and virulence phenotypes of Leishmania mexicana phosphomannomutase and dolicholphosphate-mannose synthase gene deletion mutants
- PMID: 11689705
- PMCID: PMC99981
- DOI: 10.1128/MCB.21.23.8168-8183.2001
Glycosylation defects and virulence phenotypes of Leishmania mexicana phosphomannomutase and dolicholphosphate-mannose synthase gene deletion mutants
Abstract
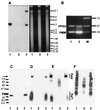
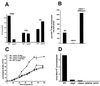
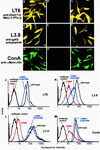
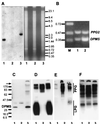
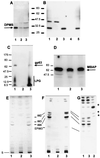
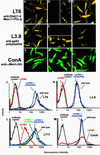
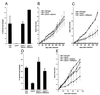
Leishmania parasites synthesize an abundance of mannose (Man)-containing glycoconjugates thought to be essential for virulence to the mammalian host and for viability. These glycoconjugates include lipophosphoglycan (LPG), proteophosphoglycans (PPGs), glycosylphosphatidylinositol (GPI)-anchored proteins, glycoinositolphospholipids (GIPLs), and N-glycans. A prerequisite for their biosynthesis is an ample supply of the Man donors GDP-Man and dolicholphosphate-Man. We have cloned from Leishmania mexicana the gene encoding the enzyme phosphomannomutase (PMM) and the previously described dolicholphosphate-Man synthase gene (DPMS) that are involved in Man activation. Surprisingly, gene deletion experiments resulted in viable parasite lines lacking the respective open reading frames (DeltaPMM and DeltaDPMS), a result against expectation and in contrast to the lethal phenotype observed in gene deletion experiments with fungi. L. mexicana DeltaDPMS exhibits a selective defect in LPG, protein GPI anchor, and GIPL biosynthesis, but despite the absence of these structures, which have been implicated in parasite virulence and viability, the mutant remains infectious to macrophages and mice. By contrast, L. mexicana DeltaPMM are largely devoid of all known Man-containing glycoconjugates and are unable to establish an infection in mouse macrophages or the living animal. Our results define Man activation leading to GDP-Man as a virulence pathway in Leishmania.
Figures


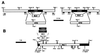

Similar articles
-
Disruption of mannose activation in Leishmania mexicana: GDP-mannose pyrophosphorylase is required for virulence, but not for viability.EMBO J. 2001 Jul 16;20(14):3657-66. doi: 10.1093/emboj/20.14.3657. EMBO J. 2001. PMID: 11447107 Free PMC article.
-
Evidence that free GPI glycolipids are essential for growth of Leishmania mexicana.EMBO J. 1999 May 17;18(10):2746-55. doi: 10.1093/emboj/18.10.2746. EMBO J. 1999. PMID: 10329621 Free PMC article.
-
Structure of Leishmania mexicana phosphomannomutase highlights similarities with human isoforms.J Mol Biol. 2006 Oct 13;363(1):215-27. doi: 10.1016/j.jmb.2006.08.023. Epub 2006 Aug 12. J Mol Biol. 2006. PMID: 16963079
-
Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species.Trends Parasitol. 2001 May;17(5):223-6. doi: 10.1016/s1471-4922(01)01895-5. Trends Parasitol. 2001. PMID: 11323305 Review.
-
Dolichol-phosphate mannose synthase: structure, function and regulation.Biochim Biophys Acta. 2008 Jun;1780(6):861-8. doi: 10.1016/j.bbagen.2008.03.005. Epub 2008 Mar 14. Biochim Biophys Acta. 2008. PMID: 18387370 Review.
Cited by
-
Deletion of transketolase triggers a stringent metabolic response in promastigotes and loss of virulence in amastigotes of Leishmania mexicana.PLoS Pathog. 2018 Mar 19;14(3):e1006953. doi: 10.1371/journal.ppat.1006953. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29554142 Free PMC article.
-
The N-terminal domain and glycosomal localization of Leishmania initial acyltransferase LmDAT are important for lipophosphoglycan synthesis.PLoS One. 2011;6(11):e27802. doi: 10.1371/journal.pone.0027802. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22114698 Free PMC article.
-
Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase.Proc Natl Acad Sci U S A. 2006 Apr 4;103(14):5502-7. doi: 10.1073/pnas.0509196103. Epub 2006 Mar 28. Proc Natl Acad Sci U S A. 2006. PMID: 16569701 Free PMC article.
-
Dissecting Leishmania infantum Energy Metabolism - A Systems Perspective.PLoS One. 2015 Sep 14;10(9):e0137976. doi: 10.1371/journal.pone.0137976. eCollection 2015. PLoS One. 2015. PMID: 26367006 Free PMC article.
-
Procyclin null mutants of Trypanosoma brucei express free glycosylphosphatidylinositols on their surface.Mol Biol Cell. 2003 Apr;14(4):1308-18. doi: 10.1091/mbc.e02-10-0694. Mol Biol Cell. 2003. PMID: 12686589 Free PMC article.
References
-
- Beverley S M, Turco S J. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 1998;6:35–40. - PubMed
-
- Boles E, Liebetrau W, Hofmann M, Zimmermann F K. A family of hexosephosphate mutases in Saccharomyces cerevisiae. Eur J Biochem. 1994;220:83–96. - PubMed
-
- Brittingham A, Morrison C J, McMaster W R, McGwire B S, Chang K P, Mosser D M. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;15:3102–3111. - PubMed
-
- Collet J, Strooban V, Pirard M, Delpierre G, Van Schaftingen E. A new class of phosphotransferases phosphorylated on an aspartate residue in an amino-terminal DXDX(T/V) motif. J Biol Chem. 1998;273:14107–14112. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
