Regulation of the Forkhead transcription factor AFX by Ral-dependent phosphorylation of threonines 447 and 451
- PMID: 11689711
- PMCID: PMC99987
- DOI: 10.1128/MCB.21.23.8225-8235.2001
Regulation of the Forkhead transcription factor AFX by Ral-dependent phosphorylation of threonines 447 and 451
Abstract
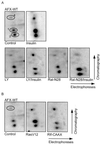
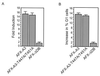
AFX is a Forkhead transcription factor that induces a G(1) cell cycle arrest via upregulation of the cell cycle inhibitor p27(Kip1). Previously we have shown that protein kinase B (PKB) phosphorylates AFX causing inhibition of AFX by nuclear exclusion. In addition, Ras, through the activation of the RalGEF-Ral pathway, induces phosphorylation of AFX. Here we show that the Ras-Ral pathway provokes phosphorylation of threonines 447 and 451 in the C terminus of AFX. A mutant protein in which both threonines are substituted for alanines (T447A/T451A) still responds to PKB-regulated nuclear-cytoplasmic shuttling, but transcriptional activity and consequent G(1) cell cycle arrest are greatly impaired. Furthermore, inhibition of the Ral signaling pathway abolishes both AFX-mediated transcription and regulation of p27(Kip1), while activation of Ral augments AFX activity. From these results we conclude that Ral-mediated phosphorylation of threonines 447 and 451 is required for proper activity of AFX-WT. Interestingly, the T447A/T451A mutation did not affect the induction of transcription and G(1) cell cycle arrest by the PKB-insensitive AFX-A3 mutant, suggesting that Ral-mediated phosphorylation plays a role in the regulation of AFX by PKB.
Figures





References
-
- Borkhardt A, Repp R, Haas O A, Leis T, Harbott J, Kreuder J, Hammermann J, Henn T, Lampert F. Cloning and characterization of AFX, the gene that fuses to MLL in acute leukemias with a t(X;11)(q13;q23) Oncogene. 1997;14:195–202. - PubMed
-
- Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. - PubMed
-
- Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
