Hierarchical phosphorylation of the translation inhibitor 4E-BP1
- PMID: 11691836
- PMCID: PMC312813
- DOI: 10.1101/gad.912401
Hierarchical phosphorylation of the translation inhibitor 4E-BP1
Abstract
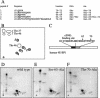
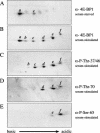
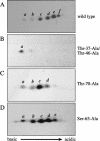
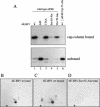
In most instances, translation is regulated at the initiation phase, when a ribosome is recruited to the 5' end of an mRNA. The eIF4E-binding proteins (4E-BPs) interdict translation initiation by binding to the translation factor eIF4E, and preventing recruitment of the translation machinery to mRNA. The 4E-BPs inhibit translation in a reversible manner. Hypophosphorylated 4E-BPs interact avidly with eIF4E, whereas 4E-BP hyperphosphorylation, elicited by stimulation of cells with hormones, cytokines, or growth factors, results in an abrogation of eIF4E-binding activity. We reported previously that phosphorylation of 4E-BP1 on Thr 37 and Thr 46 is relatively insensitive to serum deprivation and rapamycin treatment, and that phosphorylation of these residues is required for the subsequent phosphorylation of a set of unidentified serum-responsive sites. Here, using mass spectrometry, we identify the serum-responsive, rapamycin-sensitive sites as Ser 65 and Thr 70. Utilizing a novel combination of two-dimensional isoelectric focusing/SDS-PAGE and Western blotting with phosphospecific antibodies, we also establish the order of 4E-BP1 phosphorylation in vivo; phosphorylation of Thr 37/Thr 46 is followed by Thr 70 phosphorylation, and Ser 65 is phosphorylated last. Finally, we show that phosphorylation of Ser 65 and Thr 70 alone is insufficient to block binding to eIF4E, indicating that a combination of phosphorylation events is necessary to dissociate 4E-BP1 from eIF4E.
Figures



References
-
- Blachut-Okrasinska E, Bojarska E, Niedzwiecka A, Chlebicka L, Darzynkiewicz E, Stolarski R, Stepinski J, Antosiewicz JM. Stopped-flow and Brownian dynamics studies of electrostatic effects in the kinetics of binding of 7-methyl-GpppG to the protein eIF4E. Eur Biophys J. 2000;29:487–498. - PubMed
-
- Brunn GJ, Fadden P, Haystead TAJ, Lawrence JC., Jr The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem. 1997a;272:32547–32550. - PubMed
-
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997b;277:99–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
