Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival
- PMID: 11691837
- PMCID: PMC312800
- DOI: 10.1101/gad.934301
Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival
Abstract
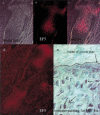
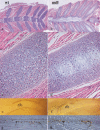
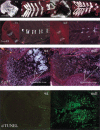
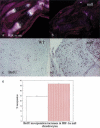
Breakdown or absence of vascular oxygen delivery is a hallmark of many common human diseases, including cancer, myocardial infarction, and stroke. The chief mediator of hypoxic response in mammalian tissues is the transcription factor hypoxia-inducible factor 1 (HIF-1), and its oxygen-sensitive component HIF-1alpha. A key question surrounding HIF-1alpha and the hypoxic response is the role of this transcription factor in cells removed from a functional vascular bed; in this regard there is evidence indicating that it can act as either a survival factor or induce growth arrest and apoptosis. To study more closely how HIF-1alpha functions in hypoxia in vivo, we used tissue-specific targeting to delete HIF-1alpha in an avascular tissue: the cartilaginous growth plate of developing bone. We show here the first evidence that the developmental growth plate in mammals is hypoxic, and that this hypoxia occurs in its interior rather than at its periphery. As a result of this developmental hypoxia, cells that lack HIF-1alpha in the interior of the growth plate die. This is coupled to decreased expression of the CDK inhibitor p57, and increased levels of BrdU incorporation in HIF-1alpha null growth plates, indicating defects in HIF-1alpha-regulated growth arrest occurs in these animals. Furthermore, we find that VEGF expression in the growth plate is regulated through both HIF-1alpha-dependent and -independent mechanisms. In particular, we provide evidence that VEGF expression is up-regulated in a HIF-1alpha-independent manner in chondrocytes surrounding areas of cell death, and this in turn induces ectopic angiogenesis. Altogether, our findings have important implications for the role of hypoxic response and HIF-1alpha in development, and in cell survival in tissues challenged by interruption of vascular flow; they also illustrate the complexities of HIF-1alpha response in vivo, and they provide new insights into mechanisms of growth plate development.
Figures



Similar articles
-
VEGF-independent cell-autonomous functions of HIF-1α regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival.J Bone Miner Res. 2012 Mar;27(3):596-609. doi: 10.1002/jbmr.1487. J Bone Miner Res. 2012. PMID: 22162090
-
Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression.Invest Ophthalmol Vis Sci. 1999 Jan;40(1):182-9. Invest Ophthalmol Vis Sci. 1999. PMID: 9888442
-
HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes.J Cell Sci. 2003 May 1;116(Pt 9):1819-26. doi: 10.1242/jcs.00385. J Cell Sci. 2003. PMID: 12665562
-
Fetal growth plate: a developmental model of cellular adaptation to hypoxia.Ann N Y Acad Sci. 2007 Nov;1117:26-39. doi: 10.1196/annals.1402.076. Ann N Y Acad Sci. 2007. PMID: 18056035 Review.
-
Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments.Curr Opin Rheumatol. 2007 Sep;19(5):457-62. doi: 10.1097/BOR.0b013e3282ba5693. Curr Opin Rheumatol. 2007. PMID: 17762611 Review.
Cited by
-
mTORC1 regulates PTHrP to coordinate chondrocyte growth, proliferation and differentiation.Nat Commun. 2016 Apr 4;7:11151. doi: 10.1038/ncomms11151. Nat Commun. 2016. PMID: 27039827 Free PMC article.
-
Distribution of Basement Membrane Molecules, Laminin and Collagen Type IV, in Normal and Degenerated Cartilage Tissues.Cartilage. 2014 Apr;5(2):123-32. doi: 10.1177/1947603513518217. Cartilage. 2014. PMID: 26069692 Free PMC article.
-
Loss of skeletal muscle HIF-1alpha results in altered exercise endurance.PLoS Biol. 2004 Oct;2(10):e288. doi: 10.1371/journal.pbio.0020288. Epub 2004 Aug 24. PLoS Biol. 2004. PMID: 15328538 Free PMC article.
-
Transcriptional, epigenetic and microRNA regulation of growth plate.Bone. 2020 Aug;137:115434. doi: 10.1016/j.bone.2020.115434. Epub 2020 May 16. Bone. 2020. PMID: 32422296 Free PMC article. Review.
-
Loss of HIF-1α in the notochord results in cell death and complete disappearance of the nucleus pulposus.PLoS One. 2014 Oct 22;9(10):e110768. doi: 10.1371/journal.pone.0110768. eCollection 2014. PLoS One. 2014. PMID: 25338007 Free PMC article.
References
-
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. - PubMed
-
- Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1α and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189–6195. - PubMed
-
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80:371–378. - PubMed
-
- Gerber H, Vu T, Ryan A, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
