Role of phosphate and calcium stores in muscle fatigue
- PMID: 11691862
- PMCID: PMC2278904
- DOI: 10.1111/j.1469-7793.2001.t01-1-00657.x
Role of phosphate and calcium stores in muscle fatigue
Abstract
Intensive activity of muscles causes a decline in performance, known as fatigue, that is thought to be caused by the effects of metabolic changes on either the contractile machinery or the activation processes. The concentration of inorganic phosphate (P(i)) in the myoplasm ([P(i)](myo)) increases substantially during fatigue and affects both the myofibrillar proteins and the activation processes. It is known that a failure of sarcoplasmic reticulum (SR) Ca(2+) release contributes to fatigue and in this review we consider how raised [P(i)](myo) contributes to this process. Initial evidence came from the observation that increasing [P(i)](myo) causes reduced SR Ca(2+) release in both skinned and intact fibres. In fatigued muscles the store of releasable Ca(2+) in the SR declines mirroring the decline in SR Ca(2+) release. In muscle fibres with inoperative creatine kinase the rise of [P(i)](myo) is absent during fatigue and the failure of SR Ca(2+) release is delayed. These results can all be explained if inorganic phosphate can move from the myoplasm into the SR during fatigue and cause precipitation of CaP(i) within the SR. The relevance of this mechanism in different types of fatigue in humans is considered.
Figures


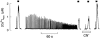
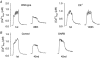
References
-
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. - PubMed
-
- Arnolda L, Brosnan J, Rajagopalan B, Radda GK. Skeletal muscle metabolism in heart failure in rats. American Journal of Physiology. 1991;261:H434–442. - PubMed
-
- Balnave CD, Allen DG. Evidence for Na+/Ca2+ exchange in intact single skeletal muscle fibers from the mouse. American Journal of Physiology. 1998;274:C940–946. - PubMed
-
- Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiologica Scandinavica. 1967;71:140–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

