Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells
- PMID: 11691874
- PMCID: PMC2278907
- DOI: 10.1111/j.1469-7793.2001.00809.x
Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells
Abstract
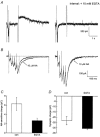
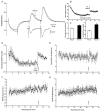
1. Whole cell recordings from CA1 pyramidal cells were performed to investigate the interaction between excitatory postsynaptic potentials (EPSPs) or currents (EPSCs), and the slow Ca(2+)-dependent K(+) current, I(sAHP). Blockers of the slow afterhyperpolarization (sAHP) such as isoprenaline (ISO) or noradrenaline (NA) reduced the hyperpolarization that followed a short train of EPSPs, and slowed the decay of summated EPSPs or EPSCs. 2. ISO/NA action on synaptic responses was observed in the absence of action potentials, but was curtailed by Ca(2+) chelation (10 mM EGTA in the electrode) and was not observed with a caesium-based recording solution. This suggests the involvement of an ISO/NA-sensitive Ca(2+)-dependent K(+) current without a requirement for regenerative spiking. 3. An ISO/NA-sensitive sAHP was observed following both NMDA and non-NMDA receptor-mediated EPSP trains in nominally zero Mg(2+) medium. Isoprenaline sensitivity was blocked by hyperpolarization during EPSPs or by isradipine, suggesting a requirement for voltage-dependent Ca(2+) influx during EPSPs. The data indicate that bursts of EPSPs can activate voltage-gated Ca(2+) channels, which trigger I(sAHP) during synaptic responses. 4. A decrease in EPSP temporal summation occurred during both spike-evoked sAHPs and persistent activation of sAHP conductance following internal dialysis with diazo-2 (2 mM). At constant membrane potential, diazo-2 caused a decrease in membrane time constant and input resistance and accelerated the rate of EPSP decay. Photolysis of diazo-2 or application of NA reduced the resting sAHP conductance, causing an increased membrane time constant and input resistance in association with an increase in EPSP half-width. 5. These results indicate that short bursts of EPSPs can activate a Ca(2+)-dependent K(+) current resembling I(sAHP), and that activation of this current reduces the postsynaptic response to high-frequency synaptic input. The findings imply that modulation of I(sAHP) can regulate synaptic efficacy and may influence the threshold for tetanus-induced synaptic plasticity.
Figures
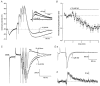

 ) and sequential application of isoprenaline (ISO, 10 μ
) and sequential application of isoprenaline (ISO, 10 μ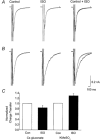



Similar articles
-
Selective shunting of the NMDA EPSP component by the slow afterhyperpolarization in rat CA1 pyramidal neurons.J Neurophysiol. 2007 May;97(5):3242-55. doi: 10.1152/jn.00422.2006. Epub 2007 Feb 28. J Neurophysiol. 2007. PMID: 17329628
-
Factors determining the efficacy of distal excitatory synapses in rat hippocampal CA1 pyramidal neurones.J Physiol. 1998 Mar 1;507 ( Pt 2)(Pt 2):441-62. doi: 10.1111/j.1469-7793.1998.441bt.x. J Physiol. 1998. PMID: 9518704 Free PMC article.
-
Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation.J Neurophysiol. 2004 Oct;92(4):2346-56. doi: 10.1152/jn.00977.2003. Epub 2004 Jun 9. J Neurophysiol. 2004. PMID: 15190096
-
Role of apamin-sensitive k(ca) channels for reticulospinal synaptic transmission to motoneuron and for the afterhyperpolarization.J Neurophysiol. 2002 Jul;88(1):289-99. doi: 10.1152/jn.2002.88.1.289. J Neurophysiol. 2002. PMID: 12091554
-
Voltage-gated sodium channels shape subthreshold EPSPs in layer 5 pyramidal neurons from rat prefrontal cortex.J Neurophysiol. 2001 Oct;86(4):1671-84. doi: 10.1152/jn.2001.86.4.1671. J Neurophysiol. 2001. PMID: 11600631
Cited by
-
CA1 pyramidal cells have diverse biophysical properties, affected by development, experience, and aging.PeerJ. 2017 Sep 19;5:e3836. doi: 10.7717/peerj.3836. eCollection 2017. PeerJ. 2017. PMID: 28948109 Free PMC article.
-
Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer's disease.Neurobiol Aging. 2011 Aug;32(8):1452-65. doi: 10.1016/j.neurobiolaging.2009.09.003. Epub 2009 Oct 14. Neurobiol Aging. 2011. PMID: 19833411 Free PMC article.
-
Chronic cocaine disrupts mesocortical learning mechanisms.Brain Res. 2015 Dec 2;1628(Pt A):88-103. doi: 10.1016/j.brainres.2015.02.003. Epub 2015 Feb 20. Brain Res. 2015. PMID: 25704202 Free PMC article. Review.
-
SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons.J Neurosci. 2007 Aug 29;27(35):9491-502. doi: 10.1523/JNEUROSCI.1106-07.2007. J Neurosci. 2007. PMID: 17728462 Free PMC article.
-
Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro.J Neurosci. 2002 Aug 15;22(16):7234-43. doi: 10.1523/JNEUROSCI.22-16-07234.2002. J Neurosci. 2002. PMID: 12177218 Free PMC article.
References
-
- Andreasen M, Lambert JDC. The excitability of CA1 pyramidal cell dendrites is modulated by a local Ca2+-dependent K+-conductance. Brain Research. 1995;698:193–203. - PubMed
-
- Bekkers JM. Distribution of slow AHP channels on hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 2000;83:1756–1759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

