Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate
- PMID: 11691880
- PMCID: PMC2278902
- DOI: 10.1111/j.1469-7793.2001.00879.x
Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate
Abstract
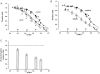
1. The effect of added inorganic phosphate (P(i), range 3-25 mM) on active tension was examined at a range of temperatures (5-30 degrees C) in chemically skinned (0.5 % Brij) rabbit psoas muscle fibres. Three types of experiments were carried out. 2. In one type of experiment, a muscle fibre was maximally activated at low temperature (5 degrees C) and its tension change was recorded during stepwise heating to high temperature in approximately 60 s. As found in previous studies, the tension increased with temperature and the normalised tension-(reciprocal) temperature relation was sigmoidal, with a half-maximal tension at 8 degrees C. In the presence of 25 mM added P(i), the temperature for half-maximal tension of the normalised curve was approximately 5 degrees C higher than in the control. The difference in the slope was small. 3. In a second type of experiment, the tension increment during a large temperature jump (from 5 to 30 degrees C) was examined during an active contraction. The relative increase of active tension on heating was significantly higher in the presence of 25 mM added P(i) (30/5 degrees C tension ratio of 6-7) than in the control with no added P(i) (tension ratio of approximately 3). 4. In a third type of experiment, the effect on the maximal Ca(2+)-activated tension of different levels of added P(i) (3-25 mM) (and P(i) mop adequate to reduce contaminating P(i) to micromolar levels) was examined at 5, 10, 20 and 30 degrees C. The tension was depressed with increased [P(i)] in a concentration-dependent manner at all temperatures, and the data could be fitted with a hyperbolic relation. The calculated maximal tension depression in excess [P(i)] was approximately 65 % of the control at 5-10 degrees C, in contrast to a maximal depression of 40 % at 20 degrees C and 30 % at 30 degrees C. 5. These experiments indicate that the active tension depression induced by P(i) in psoas fibres is temperature sensitive, the depression becoming less marked at high temperatures. A reduced P(i)-induced tension depression is qualitatively predicted by a simplified actomyosin ATPase cycle where a pre-phosphate release, force-generation step is enhanced by temperature.
Figures





Similar articles
-
Endothermic force generation, temperature-jump experiments and effects of increased [MgADP] in rabbit psoas muscle fibres.J Physiol. 2005 Sep 1;567(Pt 2):471-92. doi: 10.1113/jphysiol.2005.090084. Epub 2005 Jun 23. J Physiol. 2005. PMID: 15975981 Free PMC article.
-
An asymmetry in the phosphate dependence of tension transients induced by length perturbation in mammalian (rabbit psoas) muscle fibres.J Physiol. 2002 Aug 1;542(Pt 3):899-910. doi: 10.1113/jphysiol.2002.019471. J Physiol. 2002. PMID: 12154187 Free PMC article.
-
Force generation induced by rapid temperature jumps in intact mammalian (rat) skeletal muscle fibres.J Physiol. 2003 Apr 15;548(Pt 2):439-49. doi: 10.1113/jphysiol.2002.037143. Epub 2003 Feb 28. J Physiol. 2003. PMID: 12611915 Free PMC article.
-
Force and power generating mechanism(s) in active muscle as revealed from temperature perturbation studies.J Physiol. 2010 Oct 1;588(Pt 19):3657-70. doi: 10.1113/jphysiol.2010.194001. J Physiol. 2010. PMID: 20660565 Free PMC article. Review.
-
What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres?J Muscle Res Cell Motil. 2003;24(2-3):127-38. doi: 10.1023/a:1026093212111. J Muscle Res Cell Motil. 2003. PMID: 14609024 Review.
Cited by
-
Effect of temperature on crossbridge force changes during fatigue and recovery in intact mouse muscle fibers.PLoS One. 2013 Oct 17;8(10):e78918. doi: 10.1371/journal.pone.0078918. eCollection 2013. PLoS One. 2013. PMID: 24147145 Free PMC article.
-
Temperature change as a probe of muscle crossbridge kinetics: a review and discussion.Proc Biol Sci. 2009 Aug 7;276(1668):2685-95. doi: 10.1098/rspb.2009.0177. Epub 2009 Apr 8. Proc Biol Sci. 2009. PMID: 19364742 Free PMC article. Review.
-
Force decline during fatigue is due to both a decrease in the force per individual cross-bridge and the number of cross-bridges.J Physiol. 2011 Jul 1;589(Pt 13):3371-81. doi: 10.1113/jphysiol.2011.209874. Epub 2011 May 3. J Physiol. 2011. PMID: 21540343 Free PMC article.
-
Can inorganic phosphate explain sag during unfused tetanic contractions of skeletal muscle?Physiol Rep. 2016 Nov;4(22):e13043. doi: 10.14814/phy2.13043. Physiol Rep. 2016. PMID: 27884960 Free PMC article.
-
Mechanism of tension generation in muscle: an analysis of the forward and reverse rate constants.Biophys J. 2007 Apr 15;92(8):2865-74. doi: 10.1529/biophysj.106.101477. Epub 2007 Jan 26. Biophys J. 2007. PMID: 17259275 Free PMC article.
References
-
- Brown DES. Temperature-pressure relation in muscular contraction. In: Johnson FH, editor. Influence of Temperature on Biological Systems. Washington: American Physiological Society; 1957. pp. 83–100.
-
- Coupland ME, Puchert E, Ranatunga KW. Interactive effects of inorganic phosphate and temperature on calcium sensitivity of active force in skinned rabbit muscle fibres. Journal of Muscle Research and Cell Motility. 1999;20:820. (abstract)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

