Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani
- PMID: 11694568
- PMCID: PMC60256
- DOI: 10.1091/mbc.12.11.3307
Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani
Abstract
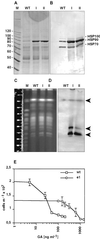
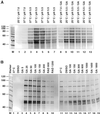
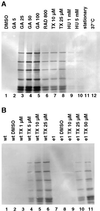
The differentiation of Leishmania parasites from the insect stage, the promastigote, toward the pathogenic mammalian stage, the amastigote, is triggered primarily by the rise in ambient temperature encountered during the insect-to-mammal transmission. We show here that inactivation of heat shock protein (Hsp) 90, with the use of the drugs geldanamycin or radicicol, mimics transmission and induces the differentiation from the promastigote to the amastigote stage. Geldanamycin also induces a growth arrest of cultured promastigotes that can be forestalled by overexpression of the cytoplasmic Hsp90. Moreover, we demonstrate that Hsp90 serves as a feedback inhibitor of the cellular heat shock response in Leishmania. Our results are consistent with Hsp90 homeostasis serving as cellular thermometer for these primitive eukaryotes, controlling both the heat shock response and morphological differentiation.
Figures




Similar articles
-
Apoptosis caused by Hsp90 inhibitor geldanamycin in Leishmania donovani during promastigote-to-amastigote transformation stage.Parasitol Res. 2009 Nov;105(6):1539-48. doi: 10.1007/s00436-009-1582-y. Epub 2009 Aug 19. Parasitol Res. 2009. PMID: 19690889
-
The heat shock protein 90 of Leishmania donovani.Med Microbiol Immunol. 2001 Nov;190(1-2):27-31. doi: 10.1007/s004300100074. Med Microbiol Immunol. 2001. PMID: 11770104
-
Leishmania donovani P23 protects parasites against HSP90 inhibitor-mediated growth arrest.Cell Stress Chaperones. 2015 Jul;20(4):673-85. doi: 10.1007/s12192-015-0595-y. Epub 2015 May 7. Cell Stress Chaperones. 2015. PMID: 25948161 Free PMC article.
-
Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs.Leukemia. 2002 Apr;16(4):455-62. doi: 10.1038/sj.leu.2402415. Leukemia. 2002. PMID: 11960322 Review.
-
An Insight to Heat Shock Protein 90: A Remedy for Multiple Problems.Curr Pharm Des. 2022;28(32):2664-2676. doi: 10.2174/1381612828666220829120630. Curr Pharm Des. 2022. PMID: 36043709 Review.
Cited by
-
Insights into Leishmania Molecules and Their Potential Contribution to the Virulence of the Parasite.Vet Sci. 2021 Feb 20;8(2):33. doi: 10.3390/vetsci8020033. Vet Sci. 2021. PMID: 33672776 Free PMC article. Review.
-
Apoptosis caused by Hsp90 inhibitor geldanamycin in Leishmania donovani during promastigote-to-amastigote transformation stage.Parasitol Res. 2009 Nov;105(6):1539-48. doi: 10.1007/s00436-009-1582-y. Epub 2009 Aug 19. Parasitol Res. 2009. PMID: 19690889
-
Control of canalization and evolvability by Hsp90.PLoS One. 2006 Dec 20;1(1):e75. doi: 10.1371/journal.pone.0000075. PLoS One. 2006. PMID: 17183707 Free PMC article.
-
The Hsp90-Sti1 interaction is critical for Leishmania donovani proliferation in both life cycle stages.Cell Microbiol. 2013 Apr;15(4):585-600. doi: 10.1111/cmi.12057. Epub 2012 Nov 20. Cell Microbiol. 2013. PMID: 23107115 Free PMC article.
-
Inhibition of HSP90 in Trypanosoma cruzi induces a stress response but no stage differentiation.Eukaryot Cell. 2002 Dec;1(6):936-43. doi: 10.1128/EC.1.6.936-943.2002. Eukaryot Cell. 2002. PMID: 12477794 Free PMC article.
References
-
- Argaman M, Aly R, Shapira M. Expression of heat shock protein 83 in Leishmania is regulated post-transcriptionally. Mol Biochem Parasitol. 1994;64:95–110. - PubMed
-
- Bates PA. Axenic amastigote culture of Leishmania amastigotes. Parasitol Today. 1993;9:143–146. - PubMed
-
- Beverley SM. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

