Pmel17 initiates premelanosome morphogenesis within multivesicular bodies
- PMID: 11694580
- PMCID: PMC60267
- DOI: 10.1091/mbc.12.11.3451
Pmel17 initiates premelanosome morphogenesis within multivesicular bodies
Abstract
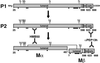
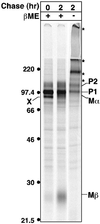
Melanosomes are tissue-specific organelles within which melanin is synthesized and stored. The melanocyte-specific glycoprotein Pmel17 is enriched in the lumen of premelanosomes, where it associates with characteristic striations of unknown composition upon which melanin is deposited. However, Pmel17 is synthesized as an integral membrane protein. To clarify its physical linkage to premelanosomes, we analyzed the posttranslational processing of human Pmel17 in pigmented and transfected nonpigmented cells. We show that Pmel17 is cleaved in a post-Golgi compartment into two disulfide-linked subunits: a large lumenal subunit, M alpha, and an integral membrane subunit, M beta. The two subunits remain associated intracellularly, indicating that detectable M alpha remains membrane bound. We have previously shown that Pmel17 accumulates on intralumenal membrane vesicles and striations of premelanosomes in pigmented cells. In transfected nonpigmented cells Pmel17 associates with the intralumenal membrane vesicles of multivesicular bodies; cells overexpressing Pmel17 also display structures resembling premelanosomal striations within these compartments. These results suggest that Pmel17 is sufficient to drive the formation of striations from within multivesicular bodies and is thus directly involved in the biogenesis of premelanosomes.
Figures







References
-
- Adema GJ, de Boer AJ, Vogel AM, Loenen WAM, Figdor CG. Molecular characterization of the melanocyte lineage-specific antigen gp100. J Biol Chem. 1994;269:20126–20133. - PubMed
-
- Berson JF, Frank DW, Calvo PA, Bieler BM, Marks MS. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J Biol Chem. 2000;275:12281–12289. - PubMed
-
- Chakraborty AK, Platt JT, Kim KK, Kwon BS, Bennett DC, Pawelek JM. Polymerization of 5,6-dihydroxyindole-2-carboxylic acid to melanin by the pmel 17/silver locus protein. Eur J Biochem. 1996;236:180–188. - PubMed
-
- Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. - PubMed
-
- Donatien PD, Orlow SJ. Interaction of melanosomal proteins with melanin. Eur J Biochem. 1995;232:159–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

