Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles
- PMID: 11694583
- PMCID: PMC60270
- DOI: 10.1091/mbc.12.11.3489
Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles
Abstract
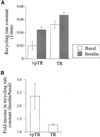
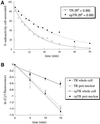
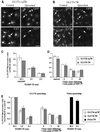
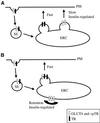
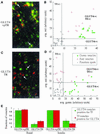
In several cell types, specific membrane proteins are retained intracellularly and rapidly redistributed to the surface in response to stimulation. In fat and muscle, the GLUT4 glucose transporter is dynamically retained because it is rapidly internalized and slowly recycled to the plasma membrane. Insulin increases the recycling of GLUT4, resulting in a net translocation to the surface. We have shown that fibroblasts also have an insulin-regulated recycling mechanism. Here we show that GLUT4 is retained within the transferrin receptor-containing general endosomal recycling compartment in Chinese hamster ovary (CHO) cells rather than being segregated to a specialized, GLUT4-recycling compartment. With the use of total internal reflection microscopy, we demonstrate that the TR and GLUT4 are transported from the pericentriolar recycling compartment in separate vesicles. These data provide the first functional evidence for the formation of distinct classes of vesicles from the recycling compartment. We propose that GLUT4 is dynamically retained within the endosomal recycling compartment in CHO cells because it is concentrated in vesicles that form more slowly than those that transport TR. In 3T3-L1 adipocytes, cells that naturally express GLUT4, we find that GLUT4 is partially segregated to a separate compartment that is inaccessible to the TR. We present a model for the formation of this specialized compartment in fat cells, based on the general mechanism described in CHO cells, which may explain the increased retention of GLUT4 and its insulin-induced translocation in fat cells.
Figures


Similar articles
-
GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps.Mol Biol Cell. 2002 Jul;13(7):2421-35. doi: 10.1091/mbc.e02-02-0071. Mol Biol Cell. 2002. PMID: 12134080 Free PMC article.
-
Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes.Biochem J. 1996 Apr 15;315 ( Pt 2)(Pt 2):487-95. doi: 10.1042/bj3150487. Biochem J. 1996. PMID: 8615819 Free PMC article.
-
Role of EHD1 and EHBP1 in perinuclear sorting and insulin-regulated GLUT4 recycling in 3T3-L1 adipocytes.J Biol Chem. 2004 Sep 17;279(38):40062-75. doi: 10.1074/jbc.M401918200. Epub 2004 Jul 9. J Biol Chem. 2004. PMID: 15247266
-
Compartment-ablation studies of GLUT4 distribution in adipocytes: evidence for multiple intracellular pools.Biochem Soc Trans. 1997 Aug;25(3):974-7. doi: 10.1042/bst0250974. Biochem Soc Trans. 1997. PMID: 9388584 Review.
-
Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles.Diabetes. 1997 Nov;46(11):1667-77. doi: 10.2337/diab.46.11.1667. Diabetes. 1997. PMID: 9356011 Review.
Cited by
-
GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif.Mol Biol Cell. 2003 Mar;14(3):973-86. doi: 10.1091/mbc.e02-06-0315. Mol Biol Cell. 2003. PMID: 12631717 Free PMC article.
-
Subcellular localization and activity of multidrug resistance proteins.Mol Biol Cell. 2003 Aug;14(8):3389-99. doi: 10.1091/mbc.e02-11-0704. Epub 2003 Apr 17. Mol Biol Cell. 2003. PMID: 12925771 Free PMC article.
-
Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway.Mol Cell Biol. 2004 Jul;24(14):6456-66. doi: 10.1128/MCB.24.14.6456-6466.2004. Mol Cell Biol. 2004. PMID: 15226445 Free PMC article.
-
Loss of AS160 Akt substrate causes Glut4 protein to accumulate in compartments that are primed for fusion in basal adipocytes.J Biol Chem. 2011 Jul 29;286(30):26287-97. doi: 10.1074/jbc.M111.253880. Epub 2011 May 24. J Biol Chem. 2011. PMID: 21613213 Free PMC article.
-
Identification of novel insulin mimetic drugs by quantitative total internal reflection fluorescence (TIRF) microscopy.Br J Pharmacol. 2014 Dec;171(23):5237-51. doi: 10.1111/bph.12845. Br J Pharmacol. 2014. PMID: 25039620 Free PMC article.
References
-
- Axelrod D, Hellen EH, Fulbright RM. Total internal reflection fluorescence. In: Lakowicz JR, editor. Topics in Fluorescence Spectroscopy. 3: Biochemical Applications. New York: Plenum Press; 1992. pp. 289–343.
-
- Bradbury NA, Bridges RJ. Role of membrane trafficking in plasma membrane solute transport. Am J Physiol. 1994;267:C1–24. - PubMed
-
- Cannon C, van Adelsberg J, Kelly S, Al-Awqati Q. Carbon-dioxide-induced exocytotic insertion of H+ pumps in turtle-bladder luminal membrane: role of cell pH and calcium. Nature. 1985;314:443–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

