Basic domains target protein subunits of the RNase MRP complex to the nucleolus independently of complex association
- PMID: 11694598
- PMCID: PMC60285
- DOI: 10.1091/mbc.12.11.3680
Basic domains target protein subunits of the RNase MRP complex to the nucleolus independently of complex association
Abstract
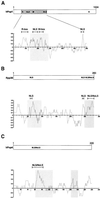
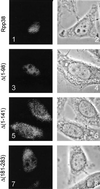
The RNase MRP and RNase P ribonucleoprotein particles both function as endoribonucleases, have a similar RNA component, and share several protein subunits. RNase MRP has been implicated in pre-rRNA processing and mitochondrial DNA replication, whereas RNase P functions in pre-tRNA processing. Both RNase MRP and RNase P accumulate in the nucleolus of eukaryotic cells. In this report we show that for three protein subunits of the RNase MRP complex (hPop1, hPop4, and Rpp38) basic domains are responsible for their nucleolar accumulation and that they are able to accumulate in the nucleolus independently of their association with the RNase MRP and RNase P complexes. We also show that certain mutants of hPop4 accumulate in the Cajal bodies, suggesting that hPop4 traverses through these bodies to the nucleolus. Furthermore, we characterized a deletion mutant of Rpp38 that preferentially associates with the RNase MRP complex, giving a first clue about the difference in protein composition of the human RNase MRP and RNase P complexes. On the basis of all available data on nucleolar localization sequences, we hypothesize that nucleolar accumulation of proteins containing basic domains proceeds by diffusion and retention rather than by an active transport process. The existence of nucleolar localization sequences is discussed.
Figures




Similar articles
-
RNA-protein interactions in the human RNase MRP ribonucleoprotein complex.RNA. 1999 Apr;5(4):512-24. doi: 10.1017/s1355838299982079. RNA. 1999. PMID: 10199568 Free PMC article.
-
Localization in the nucleolus and coiled bodies of protein subunits of the ribonucleoprotein ribonuclease P.J Cell Biol. 1999 Aug 9;146(3):559-72. doi: 10.1083/jcb.146.3.559. J Cell Biol. 1999. PMID: 10444065 Free PMC article.
-
Differential association of protein subunits with the human RNase MRP and RNase P complexes.RNA. 2006 Jul;12(7):1373-82. doi: 10.1261/rna.2293906. Epub 2006 May 24. RNA. 2006. PMID: 16723659 Free PMC article.
-
Architecture and function of the human endonucleases RNase P and RNase MRP.IUBMB Life. 2000 Apr;49(4):265-72. doi: 10.1080/15216540050033113. IUBMB Life. 2000. PMID: 10995027 Review.
-
Conserved and variable domains of RNase MRP RNA.RNA Biol. 2009 Jul-Aug;6(3):208-20. doi: 10.4161/rna.6.3.8584. Epub 2009 Jul 30. RNA Biol. 2009. PMID: 19395864 Review.
Cited by
-
An active precursor in assembly of yeast nuclear ribonuclease P.RNA. 2002 Oct;8(10):1348-60. doi: 10.1017/s1355838202027048. RNA. 2002. PMID: 12403471 Free PMC article.
-
Evolutionary comparison provides evidence for pathogenicity of RMRP mutations.PLoS Genet. 2005 Oct;1(4):e47. doi: 10.1371/journal.pgen.0010047. PLoS Genet. 2005. PMID: 16244706 Free PMC article.
-
Alterations in the intracellular level of a protein subunit of human RNase P affect processing of tRNA precursors.Nucleic Acids Res. 2003 Aug 15;31(16):4836-46. doi: 10.1093/nar/gkg691. Nucleic Acids Res. 2003. PMID: 12907726 Free PMC article.
-
Coordinated nuclear export of 60S ribosomal subunits and NMD3 in vertebrates.EMBO J. 2003 Jun 2;22(11):2841-51. doi: 10.1093/emboj/cdg249. EMBO J. 2003. PMID: 12773398 Free PMC article.
-
Footprinting analysis demonstrates extensive similarity between eukaryotic RNase P and RNase MRP holoenzymes.RNA. 2008 Aug;14(8):1558-67. doi: 10.1261/rna.1106408. Epub 2008 Jun 25. RNA. 2008. PMID: 18579867 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

