In vivo imaging of physiological angiogenesis from immature to preovulatory ovarian follicles
- PMID: 11696427
- PMCID: PMC1867040
- DOI: 10.1016/S0002-9440(10)63013-1
In vivo imaging of physiological angiogenesis from immature to preovulatory ovarian follicles
Abstract
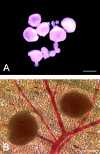
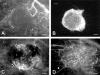
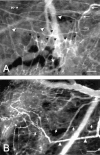
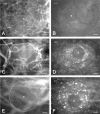
To develop a model for the study of physiological angiogenesis, we transplanted ovarian follicles onto striated muscle tissue and analyzed the process of microvascularization in vivo using repeated fluorescence microscopy. Follicles were mechanically isolated from unstimulated as well as pregnant mare's serum gonadotropin (PMSG)- or PMSG/luteinizing hormone (LH)-stimulated Syrian golden hamster ovaries and were transplanted as free grafts into dorsal skinfold chambers of untreated or synchronized hamsters. Follicles lacking thecal cell layers did not vascularize regardless whether harvested from unstimulated or PMSG-stimulated animals, but underwent granulosa cell apoptosis, as indicated in vivo by nuclear condensation and fragmentation of bisbenzimide-stained follicular tissue. In contrast, all follicles at 48 hours after PMSG treatment with a multilayered thecal shell exhibited initial signs of angiogenesis within 3 days. Vascularization was completed within 7 to 10 days, comprising a dense glomerulum-like microvascular network. Nature and extent of vascularization of follicles harvested at 72 hours after either PMSG or PMSG/LH treatment did not notably differ from each other when transplanted into the respective synchronized animals. However, follicles with PMSG/LH treatment revealed significantly larger microvessel diameters and higher capillary blood perfusion compared to follicles with sole PMSG treatment, probably reflecting the adaptation to the increased functional demand upon the LH surge. Using the unique experimental approach of ovarian follicle transplantation in the dorsal skinfold chamber of Syrian golden hamsters, we could show in vivo the developmental stage-dependent vascularization of follicular grafts with sustained potential to meet their metabolic demand by increased blood perfusion.
Figures




References
-
- Folkman J: Tumor angiogenesis. Adv Cancer Res 1985, 43:174-203 - PubMed
-
- Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 - PubMed
-
- Greenburg GB, Hunt TK: Proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol 1978, 97:353-360 - PubMed
-
- Polverini PJ, Cotran PS, Gimbrone MA, Unanue ER: Activated macrophages induce vascular proliferation. Nature 1977, 269:804-806 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

