Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site
- PMID: 11696579
- PMCID: PMC209429
- DOI: 10.1172/JCI11235
Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site
Abstract
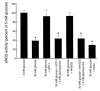
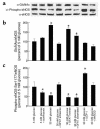
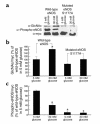
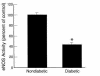
Endothelial nitric oxide synthase (eNOS) is activated by phosphorylation of serine 1177 by the protein kinase Akt/PKB. Since hyperglycemia-induced mitochondrial superoxide overproduction increases O-linked N-acetylglucosamine modification and decreases O-linked phosphorylation of the transcription factor Sp1, the effect of hyperglycemia and the hexosamine pathway on eNOS was evaluated. In bovine aortic endothelial cells, hyperglycemia inhibited eNOS activity 67%, and treatment with glucosamine had a similar effect. Hyperglycemia-associated inhibition of eNOS was accompanied by a twofold increase in O-linked N-acetylglucosamine modification of eNOS and a reciprocal decrease in O-linked serine phosphorylation at residue 1177. Both the inhibition of eNOS and the changes in its post-translational modifications were reversed by antisense inhibition of glutamine:fructose-6-phosphate amidotransferase, the rate-limiting enzyme of the hexosamine pathway, or by blocking mitochondrial superoxide overproduction with uncoupling protein-1 (UCP-1) or manganese superoxide dismutase (MnSOD). Immunoblot analysis of cells expressing myc-tagged wild-type human eNOS confirmed the reciprocal increase in O-linked N-acetylglucosamine and decrease in O-linked serine 1177 phosphorylation in response to hyperglycemia. In contrast, when myc-tagged human eNOS carried a mutation at the Akt phosphorylation site (Ser1177), O-linked N-acetylglucosamine modification was unchanged by hyperglycemia and phospho-eNOS was undetectable. Similar changes in eNOS activity and covalent modification were found in aortae from diabetic animals. Chronic impairment of eNOS activity by this mechanism may partly explain the accelerated atherosclerosis of diabetes.
Figures
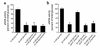




Comment in
-
More sweetness than light? A search for the causes of diabetic vasculopathy.J Clin Invest. 2001 Nov;108(10):1425-7. doi: 10.1172/JCI14508. J Clin Invest. 2001. PMID: 11714733 Free PMC article. No abstract available.
References
-
- National Diabetes Data Group. 1995. Diabetes in America. 2nd edition. National Institute of Diabetes and Digestive and Kidney Diseases. NIH publication 95-1468. Bethesda, Maryland, USA. 782 pp.
-
- Singer DE, Nathan DM, Anderson KM, Wilson PW, Evans JC. Association of HbA1c with prevalence of cardiovascular disease in the original cohort of the Framingham Study. Diabetes. 1992;41:202–208. - PubMed
-
- Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. - PubMed
-
- Jensen-Urstad KJ, Reichard PG, Rosfors JS, Lindblad LE, Jensen-Urstad MT. Early atherosclerosis is retarded by improved long-term blood glucose control in patients with IDDM. Diabetes. 1996;45:1253–1258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

