Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice
- PMID: 11696586
- PMCID: PMC2195980
- DOI: 10.1084/jem.194.9.1195
Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice
Abstract
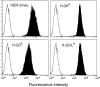
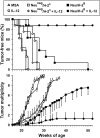
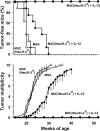
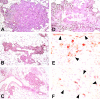
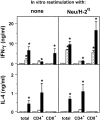
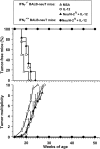
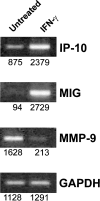
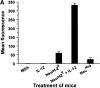
Transgenic Balb/c mice expressing the transforming rat HER-2/neu oncogene develop early and multifocal mammary carcinomas. Within the first 5 months of life the tissue-specific expression of HER-2/neu causes a progression in all their 10 mammary glands from atypical hyperplasia to invasive carcinoma. It was previously observed that chronic administration of interleukin (IL)-12 increased tumor latency, but every mouse eventually succumbed to multiple carcinomas. A significant improvement in tumor prevention was sought by administering allogeneic mammary carcinoma cells expressing HER-2/neu combined with systemic IL-12. This treatment reduced tumor incidence by 90% and more than doubled mouse lifetime. For the maximum prevention p185(neu) antigen must be expressed by allogeneic cells. IL-12 treatment strongly increased the cell vaccine efficacy. The mammary glands of mice receiving the combined treatment displayed a markedly reduced epithelial cell proliferation, angiogenesis, and HER-2/neu expression, while the few hyperplastic foci were heavily infiltrated by granulocytes, macrophages, and CD8(+) lymphocytes. Specific anti-HER-2/neu antibodies were produced and a nonpolarized activation of CD4(+) and CD8(+) cells secreting IL-4 and interferon (IFN)-gamma were evident. A central role for IFN-gamma in the preventive effect was proven by the lack of efficacy of vaccination in IFN-gamma gene knockout HER-2/neu transgenic Balb/c mice. A possible requirement for IFN-gamma is related to its effect on antibody production, in particular on IgG2a and IgG2b subclasses, that were not induced in IFN-gamma knockout HER-2/neu mice. In conclusion, our data show that an allogeneic HER-2/neu-expressing cell vaccine combined with IL-12 systemic treatment can prevent the onset of genetically determined tumors.
Figures




Similar articles
-
Immunoprevention of HER-2/neu transgenic mammary carcinoma through an interleukin 12-engineered allogeneic cell vaccine.Cancer Res. 2004 Jun 1;64(11):4001-9. doi: 10.1158/0008-5472.CAN-03-2984. Cancer Res. 2004. PMID: 15173014
-
LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c mice.Cancer Res. 2003 May 15;63(10):2518-25. Cancer Res. 2003. PMID: 12750275
-
Immunoprevention of mammary carcinoma in HER-2/neu transgenic mice is IFN-gamma and B cell dependent.J Immunol. 2004 Aug 15;173(4):2288-96. doi: 10.4049/jimmunol.173.4.2288. J Immunol. 2004. PMID: 15294941
-
Prevention by delay: nonspecific immunity elicited by IL-12 hinders Her-2/neu mammary carcinogenesis in transgenic mice.J Biol Regul Homeost Agents. 2001 Oct-Dec;15(4):351-8. J Biol Regul Homeost Agents. 2001. PMID: 11860223 Review.
-
Immunological inhibition of carcinogenesis.Cancer Immunol Immunother. 2004 Mar;53(3):204-16. doi: 10.1007/s00262-003-0483-7. Epub 2004 Jan 13. Cancer Immunol Immunother. 2004. PMID: 14722672 Free PMC article. Review.
Cited by
-
Intratumoral delivery of recombinant vaccinia virus encoding for ErbB2/Neu inhibits the growth of salivary gland carcinoma cells.J Transl Med. 2014 May 10;12:122. doi: 10.1186/1479-5876-12-122. J Transl Med. 2014. PMID: 24886178 Free PMC article.
-
Adenovirally delivered enzyme prodrug therapy with herpes simplex virus-thymidine kinase in composite tissue free flaps shows therapeutic efficacy in rat models of glioma.Plast Reconstr Surg. 2015 Feb;135(2):475-487. doi: 10.1097/PRS.0000000000000878. Plast Reconstr Surg. 2015. PMID: 25626794 Free PMC article.
-
The IL-12Rbeta2 gene functions as a tumor suppressor in human B cell malignancies.J Clin Invest. 2004 Jun;113(11):1651-9. doi: 10.1172/JCI20303. J Clin Invest. 2004. Retraction in: J Clin Invest. 2014 Jun;124(6):2807. doi: 10.1172/JCI76855. PMID: 15173892 Free PMC article. Retracted.
-
Genetic Influences in Breast Cancer Drug Resistance.Breast Cancer (Dove Med Press). 2021 Feb 9;13:59-85. doi: 10.2147/BCTT.S284453. eCollection 2021. Breast Cancer (Dove Med Press). 2021. PMID: 33603458 Free PMC article. Review.
-
Immunoprevention of non-viral cancers: challenges and strategies for early intervention.Cancer Cell Int. 2025 May 28;25(1):196. doi: 10.1186/s12935-025-03817-8. Cancer Cell Int. 2025. PMID: 40437549 Free PMC article. Review.
References
-
- Granziero, L., S. Krajewski, P. Farness, L. Yuan, M.K. Courtney, M.R. Jackson, P.A. Peterson, and A. Vitiello. 1999. Adoptive immunotherapy prevents prostate cancer in a transgenic animal model. Eur. J. Immunol. 29:1127–1138. - PubMed
-
- Cefai, D., B.W. Morrison, A. Sckell, L. Favre, M. Balli, M. Leunig, and C.D. Gimmi. 1999. Targeting HER-2/neu for active-specific immunotherapy in a mouse model of spontaneous breast cancer. Int. J. Cancer. 83:393–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

