Functional cloning of Src-like adapter protein-2 (SLAP-2), a novel inhibitor of antigen receptor signaling
- PMID: 11696592
- PMCID: PMC2195979
- DOI: 10.1084/jem.194.9.1263
Functional cloning of Src-like adapter protein-2 (SLAP-2), a novel inhibitor of antigen receptor signaling
Abstract
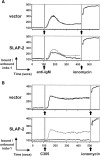
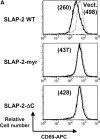
In an effort to identify novel therapeutic targets for autoimmunity and transplant rejection, we developed and performed a large-scale retroviral-based functional screen to select for proteins that inhibit antigen receptor-mediated activation of lymphocytes. In addition to known regulators of antigen receptor signaling, we identified a novel adaptor protein, SLAP-2 which shares 36% sequence similarity with the known Src-like adaptor protein, SLAP. Similar to SLAP, SLAP-2 is predominantly expressed in hematopoietic cells. Overexpression of SLAP-2 in B and T cell lines specifically impaired antigen receptor-mediated signaling events, including CD69 surface marker upregulation, nuclear factor of activated T cells (NFAT) promoter activation and calcium influx. Signaling induced by phorbol myristate acetate (PMA) and ionomycin was not significantly reduced, suggesting SLAP-2 functions proximally in the antigen receptor signaling cascade. The SLAP-2 protein contains an NH2-terminal myristoylation consensus sequence and SH3 and SH2 Src homology domains, but lacks a tyrosine kinase domain. In antigen receptor-stimulated cells, SLAP-2 associated with several tyrosine phosphorylated proteins, including the ubiquitin ligase Cbl. Deletion of the COOH terminus of SLAP-2 blocked function and abrogated its association with Cbl. Mutation of the putative myristoylation site of SLAP-2 compromised its inhibitory activity and impaired its localization to the membrane compartment. Our identification of the negative regulator SLAP-2 demonstrates that a retroviral-based screening strategy may be an efficient way to identify and characterize the function of key components of many signal transduction systems.
Figures

















Similar articles
-
Role of SRC-like adaptor protein (SLAP) in immune and malignant cell signaling.Cell Mol Life Sci. 2015 Jul;72(13):2535-44. doi: 10.1007/s00018-015-1882-6. Epub 2015 Mar 13. Cell Mol Life Sci. 2015. PMID: 25772501 Free PMC article. Review.
-
Functional cooperation between c-Cbl and Src-like adaptor protein 2 in the negative regulation of T-cell receptor signaling.Mol Cell Biol. 2002 Jun;22(12):4241-55. doi: 10.1128/MCB.22.12.4241-4255.2002. Mol Cell Biol. 2002. PMID: 12024036 Free PMC article.
-
A novel Src homology 2 domain-containing molecule, Src-like adapter protein-2 (SLAP-2), which negatively regulates T cell receptor signaling.J Biol Chem. 2002 May 24;277(21):19131-8. doi: 10.1074/jbc.M110318200. Epub 2002 Mar 12. J Biol Chem. 2002. PMID: 11891219
-
Src-like adaptor protein (SLAP) is a negative regulator of T cell receptor signaling.J Exp Med. 2000 Feb 7;191(3):463-74. doi: 10.1084/jem.191.3.463. J Exp Med. 2000. PMID: 10662792 Free PMC article.
-
RTK SLAP down: the emerging role of Src-like adaptor protein as a key player in receptor tyrosine kinase signaling.Cell Signal. 2015 Feb;27(2):267-74. doi: 10.1016/j.cellsig.2014.11.010. Epub 2014 Nov 18. Cell Signal. 2015. PMID: 25446260 Review.
Cited by
-
Role of SRC-like adaptor protein (SLAP) in immune and malignant cell signaling.Cell Mol Life Sci. 2015 Jul;72(13):2535-44. doi: 10.1007/s00018-015-1882-6. Epub 2015 Mar 13. Cell Mol Life Sci. 2015. PMID: 25772501 Free PMC article. Review.
-
Long Noncoding RNAs and mRNA Regulation in Peripheral Blood Mononuclear Cells of Patients with Chronic Obstructive Pulmonary Disease.Mediators Inflamm. 2018 Mar 13;2018:7501851. doi: 10.1155/2018/7501851. eCollection 2018. Mediators Inflamm. 2018. PMID: 29725270 Free PMC article.
-
Combining Landscape Genomics and Ecological Modelling to Investigate Local Adaptation of Indigenous Ugandan Cattle to East Coast Fever.Front Genet. 2018 Oct 3;9:385. doi: 10.3389/fgene.2018.00385. eCollection 2018. Front Genet. 2018. PMID: 30333851 Free PMC article.
-
Mechanism of upregulation of the inhibitory regulator, src-like adaptor protein (SLAP), by glucocorticoids in mast cells.Mol Immunol. 2009 Jan;46(3):492-7. doi: 10.1016/j.molimm.2008.10.011. Epub 2008 Nov 25. Mol Immunol. 2009. PMID: 19036452 Free PMC article.
-
Correlation of gene expression and genome mutation in single B-cells.PLoS One. 2013 Jun 28;8(6):e67624. doi: 10.1371/journal.pone.0067624. Print 2013. PLoS One. 2013. PMID: 23840752 Free PMC article.
References
-
- Weiss, A., and D.R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell. 76:263–274. - PubMed
-
- DeFranco, A.L. 1997. The complexity of signaling pathways activated by the BCR. Curr. Opin. Immunol. 9:296–308. - PubMed
-
- Bolen, J.B. 1995. Protein tyrosine kinases in the initiation of antigen receptor signaling. Curr. Opin. Immunol. 7:306–311. - PubMed
-
- Kurosaki, T. 2000. Functional dissection of BCR signaling pathways. Curr. Opin. Immunol. 12:276–281. - PubMed
-
- Kelly, M.E., and A.C. Chan. 2000. Regulation of B cell function by linker proteins. Curr. Opin. Immunol. 12:267–275. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

