The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors
- PMID: 11696595
- PMCID: PMC2195969
- DOI: 10.1084/jem.194.9.1299
The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors
Abstract
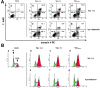
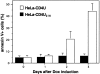
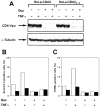
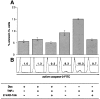
Human immunodeficiency virus (HIV) type 1 Vpu is an integral membrane protein with a unique affinity for betaTrCP (TrCP), a key member of the SkpI-Cullin-F-box E3 ubiquitin ligase complex that is involved in the regulated degradation of cellular proteins, including IkappaB. Remarkably, Vpu is resistant to TrCP-mediated degradation and competitively inhibits TrCP-dependent degradation of IkappaB, resulting in the suppression of nuclear factor (NF)-kappaB activity in Vpu-expressing cells. We now report that Vpu, through its interaction with TrCP, potently contributes to the induction of apoptosis in HIV-infected T cells. Vpu-induced apoptosis is specific and independent of other viral proteins. Mutation of a TrCP-binding motif in Vpu abolishes its apoptogenic property, demonstrating a close correlation between this property of Vpu and its ability to inhibit NF-kappaB activity. The involvement of NF-kappaB in Vpu-induced apoptosis is further supported by the finding that the levels of antiapoptotic factors Bcl-xL, A1/Bfl-1, and TNF receptor-associated factor (TRAF)1, all of which are expressed in an NF-kappaB-dependent manner, are reduced and, at the same time, levels of active caspase-3 are elevated. Thus, Vpu induces apoptosis through activation of the caspase pathway by way of inhibiting the NF-kappaB-dependent expression of antiapoptotic genes.
Figures




References
-
- Badley, A.D., A.A. Pilon, A. Landay, and D.H. Lynch. 2000. Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 96:2951–2964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

