Hyper immunoglobulin E response in mice with monoclonal populations of B and T lymphocytes
- PMID: 11696599
- PMCID: PMC2195981
- DOI: 10.1084/jem.194.9.1349
Hyper immunoglobulin E response in mice with monoclonal populations of B and T lymphocytes
Abstract
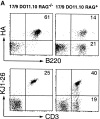
A key event in the pathogenesis of allergies is the production of antibodies of the immunoglobulin (Ig)E class. In normal individuals the levels of IgE are tightly regulated, as illustrated by the low serum IgE concentration. In addition, multiple immunizations are usually required to generate detectable IgE responses in normal experimental animals. To define the parameters that regulate IgE production in vivo, we generated mice bearing monoclonal populations of B and T lymphocytes specific for influenza virus hemagglutinin (HA) and chicken ovalbumin (OVA), respectively. A single immunization of the monoclonal mice with the cross-linked OVA-HA antigen led to serum IgE levels that reached 30-200 microg/ml. This unusually high IgE response was prevented by the infusion of regulatory alpha/beta CD4(+) T cells belonging to both CD25(+) and CD25(-) subpopulations. The regulation by the infused T cells impeded the development of fully competent OVA-specific effector/memory Th2 lymphocytes without inhibiting the initial proliferative response of T cells or promoting activation-induced cell death. Our results indicate that hyper IgE responses do not occur in normal individuals due to the presence of regulatory T cells, and imply that the induction of regulatory CD4(+) T cells could be used for the prevention of atopy.
Figures




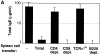
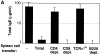
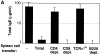








References
-
- Corry, D.B., and F. Kheradmand. 1999. Induction and regulation of the IgE response. Nature. 402:B18–B23. - PubMed
-
- Chilosi, M., F. Facchetti, L.D. Notarangelo, S. Romagnani, G. Del Prete, F. Almerigogna, M. De Carli, and G. Pizzolo. 1996. CD30 cell expression and abnormal soluble CD30 serum accumulation in Omenn's syndrome: evidence for a T helper 2-mediated condition. Eur. J. Immunol. 26:329–334. - PubMed
-
- Notarangelo, L.D., A. Villa, and K. Schwarz. 1999. RAG and RAG defects. Curr. Opin. Immunol. 11:435–442. - PubMed
-
- Grimbacher, B., S.M. Holland, J.I. Gallin, F. Greenberg, S.C. Hill, H.L. Malech, J.A. Miller, A.C. O'Connell, and J.M. Puck. 1999. Hyper-IgE syndrome with recurrent infections—an autosomal dominant multisystem disorder. N. Engl. J. Med. 340:692–702. - PubMed
-
- Lucey, D.R., R.A. Zajac, G.P. Melcher, C.A. Butzin, and R.N. Boswell. 1990. Serum IgE levels in 622 persons with human immunodeficiency virus infection: IgE elevation with marked depletion of CD4+ T-cells. AIDS Res. Hum. Retrovir. 6:427–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

