Regulation of taurine transporter expression by NO in cultured human retinal pigment epithelial cells
- PMID: 11698241
- PMCID: PMC4637984
- DOI: 10.1152/ajpcell.2001.281.6.C1825
Regulation of taurine transporter expression by NO in cultured human retinal pigment epithelial cells
Abstract
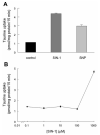
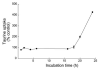
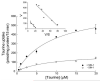
Taurine is actively transported at the retinal pigment epithelial (RPE) apical membrane in an Na(+)- and Cl(-)-dependent manner. Diabetes may alter the function of the taurine transporter. Because nitric oxide (NO) is a molecule implicated in the pathogenesis of diabetes, we asked whether NO would alter the activity of the taurine transporter in cultured ARPE-19 cells. The activity of the transporter was stimulated in the presence of the NO donor 3-morpholinosydnonimine. The stimulatory effects of 3-morpholinosydnonimine were not observed during the initial 16-h treatment; however, stimulation of taurine uptake was elevated dramatically above control values with 20- and 24-h treatments. Kinetic analysis revealed that the stimulation was associated with an increase in the maximal velocity of the transporter with no significant change in the substrate affinity. The NO-induced increase in taurine uptake was inhibited by actinomycin D and cycloheximide. RT-PCR analysis and nuclear run-on assays provided evidence for upregulation of the transporter gene. This study provides the first evidence of an increase in taurine transporter gene expression in human RPE cells cultured under conditions of elevated levels of NO.
Figures




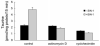


Similar articles
-
Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and müller cells.Invest Ophthalmol Vis Sci. 2004 Feb;45(2):694-701. doi: 10.1167/iovs.03-0503. Invest Ophthalmol Vis Sci. 2004. PMID: 14744916 Free PMC article.
-
Cholera toxin enhances taurine uptake in cultures of human retinal pigment epithelial cells.Curr Eye Res. 1996 Mar;15(3):229-36. doi: 10.3109/02713689609007616. Curr Eye Res. 1996. PMID: 8654102
-
Molecular identity and calmodulin-mediated regulation of the taurine transporter in a human retinal pigment epithelial cell line.Curr Eye Res. 1994 Jul;13(7):523-9. doi: 10.3109/02713689408999884. Curr Eye Res. 1994. PMID: 7924416
-
Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells.Exp Neurol. 2012 Jan;233(1):154-62. doi: 10.1016/j.expneurol.2011.09.010. Epub 2011 Sep 17. Exp Neurol. 2012. PMID: 21952043 Free PMC article.
-
Expression and regulation of the taurine transporter in cultured cell lines of human origin.Adv Exp Med Biol. 1994;359:51-7. doi: 10.1007/978-1-4899-1471-2_6. Adv Exp Med Biol. 1994. PMID: 7887288 Review. No abstract available.
Cited by
-
Correlation of taurine transport with membrane lipid composition and peroxidation in DHA-enriched Caco-2 cells.J Membr Biol. 2009 Apr;228(3):141-50. doi: 10.1007/s00232-009-9166-4. Epub 2009 Apr 7. J Membr Biol. 2009. PMID: 19350314
-
Effects of taurine on nitric oxide and 3-nitrotyrosine levels in spleen during endotoxemia.Neurochem Res. 2011 Nov;36(11):1978-83. doi: 10.1007/s11064-011-0521-3. Epub 2011 Jun 15. Neurochem Res. 2011. PMID: 21674239
-
Taurine: A Maternally Derived Nutrient Linking Mother and Offspring.Metabolites. 2022 Mar 5;12(3):228. doi: 10.3390/metabo12030228. Metabolites. 2022. PMID: 35323671 Free PMC article. Review.
-
Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry.J Am Soc Nephrol. 2004 Mar;15(3):663-73. doi: 10.1097/01.asn.0000113553.62380.f5. J Am Soc Nephrol. 2004. PMID: 14978168 Free PMC article.
-
The relationship between taurine and 3-nitrotyrosine level of hepatocytes in experimental endotoxemia.Neurochem Res. 2007 Nov;32(11):1965-8. doi: 10.1007/s11064-007-9395-9. Epub 2007 Jun 15. Neurochem Res. 2007. PMID: 17570060
References
-
- Abu el Asrar AM, Maimone D, Morse PH, Gregory S, Reder AT. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1992;114:731–736. - PubMed
-
- Bank N, Aynedjian HS. Role of EDRF (nitric oxide) in diabetic renal hyperfiltration. Kidney Int. 1993;43:1306–1312. - PubMed
-
- Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Regulation of taurine transport in human colon carcinoma cell lines (HT-29 and Caco-2) by protein kinase C. Am J Physiol Gastrointest Liver Physiol. 1993;264:G939–G946. - PubMed
-
- Bridges CC, Kekuda R, Wang H, Prasad P, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:47–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

