Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms
- PMID: 11698377
- PMCID: PMC95529
- DOI: 10.1128/JB.183.23.6875-6884.2001
Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms
Abstract
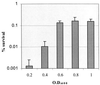
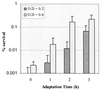
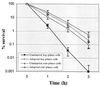
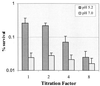
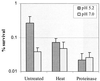
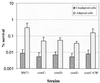
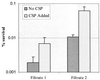
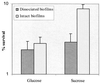
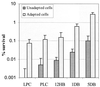
Streptococcus mutans normally colonizes dental biofilms and is regularly exposed to continual cycles of acidic pH during ingestion of fermentable dietary carbohydrates. The ability of S. mutans to survive at low pH is an important virulence factor in the pathogenesis of dental caries. Despite a few studies of the acid adaptation mechanism of this organism, little work has focused on the acid tolerance of S. mutans growing in high-cell-density biofilms. It is unknown whether biofilm growth mode or high cell density affects acid adaptation by S. mutans. This study was initiated to examine the acid tolerance response (ATR) of S. mutans biofilm cells and to determine the effect of cell density on the induction of acid adaptation. S. mutans BM71 cells were first grown in broth cultures to examine acid adaptation associated with growth phase, cell density, carbon starvation, and induction by culture filtrates. The cells were also grown in a chemostat-based biofilm fermentor for biofilm formation. Adaptation of biofilm cells to low pH was established in the chemostat by the acid generated from excess glucose metabolism, followed by a pH 3.5 acid shock for 3 h. Both biofilm and planktonic cells were removed to assay percentages of survival. The results showed that S. mutans BM71 exhibited a log-phase ATR induced by low pH and a stationary-phase acid resistance induced by carbon starvation. Cell density was found to modulate acid adaptation in S. mutans log-phase cells, since pre-adapted cells at a higher cell density or from a dense biofilm displayed significantly higher resistance to the killing pH than the cells at a lower cell density. The log-phase ATR could also be induced by a neutralized culture filtrate collected from a low-pH culture, suggesting that the culture filtrate contained an extracellular induction component(s) involved in acid adaptation in S. mutans. Heat or proteinase treatment abolished the induction by the culture filtrate. The results also showed that mutants defective in the comC, -D, or -E genes, which encode a quorum sensing system essential for cell density-dependent induction of genetic competence, had a diminished log-phase ATR. Addition of synthetic competence stimulating peptide (CSP) to the comC mutant restored the ATR. This study demonstrated that cell density and biofilm growth mode modulated acid adaptation in S. mutans, suggesting that optimal development of acid adaptation in this organism involves both low pH induction and cell-cell communication.
Figures
References
-
- Bowden G H, Hamilton I R. Competition between Streptococcus mutans and Lactobacillus casei in mixed continuous culture. Oral Microbiol Immunol. 1989;4:57–64. - PubMed
-
- Bowden G H, Hamilton I R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and “S. mitior” growing in continuous culture. Can J Microbiol. 1987;33:824–827. - PubMed
-
- Bradshaw D J, McKee A S, Marsh P D. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989;68:1298–1302. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

