Spontaneous activity in developing ferret visual cortex in vivo
- PMID: 11698602
- PMCID: PMC6762264
- DOI: 10.1523/JNEUROSCI.21-22-08906.2001
Spontaneous activity in developing ferret visual cortex in vivo
Abstract
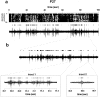
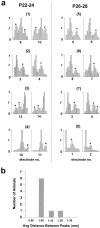
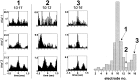
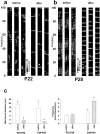
Multi-electrode extracellular recordings in area 17 of awake behaving ferrets were conducted to characterize the pattern of spontaneous activity in the developing visual cortex before eye opening. A linear array of 16 microwire electrodes was used to record extracellular neuronal activity across a 3.2 mm strip of visual cortex between postnatal days 22 and 28. Whereas synchronous bursts of activity were observed at all recording sites, cross-correlation analysis revealed that the timing of spike activity at all electrodes was not precisely correlated. Correlated activity between cortical sites exhibited a patchy organization having long-range components. Long-range correlated activity was observed between cortical patches that were separated by a mean distance of 1 mm. The spatial pattern of correlated activity persisted during transient lateral geniculate nucleus (LGN) activity block, indicating that long-range correlated activity is generated by intrinsic circuits within the cortex, independent of LGN input activity. These results demonstrate an innate patchy organization of correlated spontaneous activity within the cortex during the early development of cortical functional and anatomical organization.
Figures


References
-
- Caríc D, Price DJ. Evidence that the lateral geniculate nucleus regulates the normal development of visual corticocortical projections in the cat. Exp Neurol. 1999;156:353–362. - PubMed
-
- Crowley JC, Katz LC. Development of ocular dominance columns in the absence of retinal input. Nat Neuroscience. 1999;2:1125–1129. - PubMed
-
- Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
