Sorting of internalized neurotrophins into an endocytic transcytosis pathway via the Golgi system: Ultrastructural analysis in retinal ganglion cells
- PMID: 11698603
- PMCID: PMC6762282
- DOI: 10.1523/JNEUROSCI.21-22-08915.2001
Sorting of internalized neurotrophins into an endocytic transcytosis pathway via the Golgi system: Ultrastructural analysis in retinal ganglion cells
Abstract
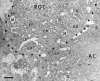
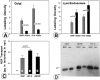
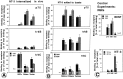
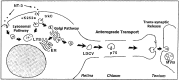
Subcellular pathways and accumulation of internalized radiolabeled neurotrophins NGF, BDNF, and NT-3 were examined in retinal ganglion cells (RGCs) of chick embryos by using quantitative electron microscopic autoradiography. All three neurotrophins accumulated in endosomes and multivesicular bodies. BDNF and NGF also concentrated at the plasma membrane, whereas NT-3 accumulated transiently in the Golgi system. The enhanced targeting of NT-3 to the Golgi system correlated with the anterograde axonal transport of this neurotrophin. Anterograde transport of NT-3, but not its internalization, was significantly attenuated by the tyrosine kinase (trk) inhibitor K252a. Abolishment of trk activity with K252a shifted NT-3 (and BDNF) away from the Golgi system and into a lysosomal pathway, indicating that trk activity regulated sorting of the ligand-receptor complex. Cross-linking of neurotrophins and immunoprecipitation with antibodies to the neurotrophin receptors p75, trkA, trkB, and trkC showed that the large majority of exogenous, receptor-bound NT-3 was bound to trkC in RGC somata, but during anterograde transport in the optic nerve most receptor-bound NT-3 was associated with p75, and after arrival and release in the optic tectum transferred to presumably postsynaptic trkC. These results reveal remarkable and unexpected differences in the intracellular pathways and fates of different neurotrophins within the same cell type. They provide first evidence for an endocytic pathway of internalized neurotrophic factors via the Golgi system before anterograde transport and transcytosis. The results challenge the belief that after internalization all neurotrophins are rapidly degraded in lysosomes.
Figures




References
-
- Alderson RF, Curtis R, Alterman AL, Lindsay RM, DiStefano PS. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and Schwann cells in vitro. Brain Res. 2000;871:210–222. - PubMed
-
- Barker PA, Murphy RA. The nerve growth factor receptor: a multicomponent system that mediates the actions of the neurotrophin family of proteins. Mol Cell Biochem. 1992;110:1–15. - PubMed
-
- Bartlett SE, Reynolds AJ, Weible M, Heydon K, Hendry IA. In sympathetic but not sensory neurones, phosphoinositide-3 kinase is important for NGF-dependent survival and the retrograde transport of 125I-betaNGF. Brain Res. 1997;761:257–262. - PubMed
-
- Bernd P, Greene LA. Association of [125I] nerve growth factor with PC12 pheochromocytoma cells. Evidence for internalization via high-affinity receptors only and for long-term regulation by nerve growth factor of both high- and low-affinity receptors. J Biol Chem. 1984;259:15509–15516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
