PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine
- PMID: 11698646
- PMCID: PMC61133
- DOI: 10.1073/pnas.231486598
PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine
Abstract
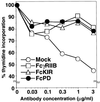
PD-1 is an immunoreceptor that belongs to the immunoglobulin (Ig) superfamily and contains two tyrosine residues in the cytoplasmic region. Studies on PD-1-deficient mice have shown that PD-1 plays critical roles in establishment and/or maintenance of peripheral tolerance, but the mode of action is totally unknown. To study the molecular mechanism for negative regulation of lymphocytes through the PD-1 receptor, we generated chimeric molecules composed of the IgG Fc receptor type IIB (Fc gamma RIIB) extracellular region and the PD-1 cytoplasmic region and expressed them in a B lymphoma cell line, IIA1.6. Coligation of the cytoplasmic region of PD-1 with the B cell receptor (BCR) in IIA1.6 transformants inhibited BCR-mediated growth retardation, Ca(2+) mobilization, and tyrosine phosphorylation of effector molecules, including Ig beta, Syk, phospholipase C-gamma 2 (PLC gamma 2), and ERK1/2, whereas phosphorylation of Lyn and Dok was not affected. Mutagenesis studies indicated that these inhibitory effects do not require the N-terminal tyrosine in the immunoreceptor tyrosine-based inhibitory motif-like sequence, but do require the other tyrosine residue in the C-terminal tail. This tyrosine was phosphorylated and recruited src homology 2-domain-containing tyrosine phosphatase 2 (SHP-2) on coligation of PD-1 with BCR. These results show that PD-1 can inhibit BCR signaling by recruiting SHP-2 to its phosphotyrosine and dephosphorylating key signal transducers of BCR signaling.
Figures




References
-
- Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Genomics. 1994;23:704–706. - PubMed
-
- Bolland S, Ravetch J-V. Adv Immunol. 1999;72:149–177. - PubMed
-
- Unkeless J-C, Jin J. Curr Opin Immunol. 1997;9:338–343. - PubMed
-
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Int Immunol. 1996;8:765–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

