Regulation of cyclic peptide biosynthesis in a plant pathogenic fungus by a novel transcription factor
- PMID: 11698648
- PMCID: PMC61187
- DOI: 10.1073/pnas.231491298
Regulation of cyclic peptide biosynthesis in a plant pathogenic fungus by a novel transcription factor
Abstract
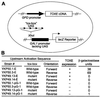
Strains of the filamentous fungus Cochliobolus carbonum that produce the host-selective compound HC-toxin, a cyclic tetrapeptide, are highly virulent on certain genotypes of maize (Zea mays L.). Production of HC-toxin is under the control of a complex locus, TOX2, which is composed of at least seven linked and duplicated genes that are present only in toxin-producing strains of C. carbonum. One of these genes, TOXE, was earlier shown to be required for the expression of the other TOX2 genes. TOXE has four ankyrin repeats and a basic region similar to those found in basic leucine zipper (bZIP) proteins, but lacks any apparent leucine zipper. Here we show that TOXE is a DNA-binding protein that recognizes a ten-base motif (the "tox-box") without dyad symmetry that is present in the promoters of all of the known TOX2 genes. Both the basic region and the ankyrin repeats are involved in DNA binding. A region of TOXE that includes the first ankyrin repeat is necessary and sufficient for transcriptional activation in yeast. The data indicate that TOXE is the prototype of a new family of transcription factor, so far found only in plant-pathogenic fungi. TOXE plays a specific regulatory role in HC-toxin production and, therefore, pathogenicity by C. carbonum.
Figures
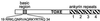





Comment in
-
Tox-boxes, fungal secondary metabolites, and plant disease.Proc Natl Acad Sci U S A. 2001 Dec 4;98(25):14187-8. doi: 10.1073/pnas.261573598. Proc Natl Acad Sci U S A. 2001. PMID: 11734635 Free PMC article. No abstract available.
References
-
- Hammond-Kosack K, Jones J D G. In: Biochemistry and Molecular Biology of Plants. Buchanan B B, Gruissem W, Jones R L, editors. Rockville, MD: Am. Soc. Plant Biologists; 2000. pp. 1102–1156.
-
- Kohmoto K, Otani H. Experientia. 1991;47:755–764. - PubMed
-
- Johal G S, Briggs S P. Science. 1992;258:985–987. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

