Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway
- PMID: 11698663
- PMCID: PMC61152
- DOI: 10.1073/pnas.241411198
Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway
Abstract
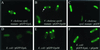
Bacteria have long been thought of as little more than sacks of homogeneously distributed enzymes. However, recent cytological studies indicate that bacteria are compartmentalized with proteins involved in processes such as cell division, motility, chemotaxis, and development located at distinct sites. We have used the green fluorescent protein as a reporter to determine the cellular distribution of the extracellular protein secretion (eps)-encoded type II secretion complex responsible for extracellular secretion of cholera toxin and hemagglutinin/protease in Vibrio cholerae. Real-time monitoring of green fluorescent protein fused to EpsM in living cells indicated that, like the single polar flagellum, the Eps complex is located at the old pole after cell division. Eps-dependent protease secretion was also visualized in single cells by fluorescence microscopy by using intramolecularly quenched casein. This analysis demonstrated that active protease secretion is focused at the poles and colocalizes with the site of the polar Eps apparatus. These results suggest that the type II secretion complex is responsible for directed delivery of virulence factors during cholera pathogenesis.
Figures





Similar articles
-
The crystal structure of the periplasmic domain of the type II secretion system protein EpsM from Vibrio cholerae: the simplest version of the ferredoxin fold.J Mol Biol. 2004 Apr 30;338(3):585-96. doi: 10.1016/j.jmb.2004.01.064. J Mol Biol. 2004. PMID: 15081815
-
The X-ray structure of the type II secretion system complex formed by the N-terminal domain of EpsE and the cytoplasmic domain of EpsL of Vibrio cholerae.J Mol Biol. 2005 May 13;348(4):845-55. doi: 10.1016/j.jmb.2005.02.061. J Mol Biol. 2005. PMID: 15843017
-
The structure of the cytoplasmic domain of EpsL, an inner membrane component of the type II secretion system of Vibrio cholerae: an unusual member of the actin-like ATPase superfamily.J Mol Biol. 2004 Nov 26;344(3):619-33. doi: 10.1016/j.jmb.2004.09.062. J Mol Biol. 2004. PMID: 15533433
-
Chemotaxis in Vibrio cholerae.FEMS Microbiol Lett. 2004 Oct 1;239(1):1-8. doi: 10.1016/j.femsle.2004.08.039. FEMS Microbiol Lett. 2004. PMID: 15451094 Review.
-
Generating and exploiting polarity in bacteria.Science. 2002 Dec 6;298(5600):1942-6. doi: 10.1126/science.1072163. Science. 2002. PMID: 12471245 Review.
Cited by
-
Membrane topology mapping of the Na+-pumping NADH: quinone oxidoreductase from Vibrio cholerae by PhoA-green fluorescent protein fusion analysis.J Bacteriol. 2006 Dec;188(24):8343-51. doi: 10.1128/JB.01383-06. Epub 2006 Oct 13. J Bacteriol. 2006. PMID: 17041063 Free PMC article.
-
Docking and assembly of the type II secretion complex of Vibrio cholerae.J Bacteriol. 2009 May;191(9):3149-61. doi: 10.1128/JB.01701-08. Epub 2009 Feb 27. J Bacteriol. 2009. PMID: 19251862 Free PMC article.
-
The selective value of bacterial shape.Microbiol Mol Biol Rev. 2006 Sep;70(3):660-703. doi: 10.1128/MMBR.00001-06. Microbiol Mol Biol Rev. 2006. PMID: 16959965 Free PMC article. Review.
-
Specificity of the type II secretion systems of enterotoxigenic Escherichia coli and Vibrio cholerae for heat-labile enterotoxin and cholera toxin.J Bacteriol. 2010 Apr;192(7):1902-11. doi: 10.1128/JB.01542-09. Epub 2010 Jan 22. J Bacteriol. 2010. PMID: 20097854 Free PMC article.
-
Quantification and Surface Localization of the Hemolysin A Type I Secretion System at the Endogenous Level and under Conditions of Overexpression.Appl Environ Microbiol. 2022 Feb 8;88(3):e0189621. doi: 10.1128/AEM.01896-21. Epub 2021 Dec 1. Appl Environ Microbiol. 2022. PMID: 34851699 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

