Impact of reference-based pricing of nitrates on the use and costs of anti-anginal drugs
- PMID: 11699696
- PMCID: PMC81535
Impact of reference-based pricing of nitrates on the use and costs of anti-anginal drugs
Abstract
Background: Reference-based pricing limits reimbursement for a group of drugs that are deemed therapeutically equivalent to the cost of the lowest-priced product within that group. We estimated the effect of reference-based pricing of nitrate drugs used for long-term prophylaxis on prescribing of and expenditures on nitrates and other anti-anginal drugs dispensed to senior citizens in British Columbia.
Methods: We assessed trends in the monthly volume of prescriptions of anti-anginal drugs and the associated drug ingredient cost paid by the province's publicly funded drug subsidy program, Pharmacare, and by the patients themselves for the period April 1994 to May 1999. Trends in monthly rates of nitrate expenditures per 100,000 senior citizens before the introduction of reference-based pricing were extrapolated to infer what expenditures would have been without the policy.
Results: During the 3 1/2 years after reference-based pricing was introduced, Pharmacare expenditures on nitrates prescribed to senior citizens declined by $14.9 million (95% confidence interval $10.7 to $19.1 million). Most of these savings were due to the lower prices that Pharmacare paid for sustained-release nitroglycerin tablets and the nitroglycerin patch, which were the 2 most frequently prescribed nitrates before the introduction of reference-based pricing; $1.2 million (8%) of the savings represented expenditures by senior citizens who purchased drugs that were only partially reimbursed. There were no compensatory increases in expenditures for other anti-anginal drugs. Use of sublingual nitroglycerin--a marker for deteriorating health in patients with angina--did not increase after the introduction of reference-based pricing. The nitroglycerin patch is now the most frequently prescribed nitrate, owing to the fact that Pharmacare resumed the provision of full subsidies for the drug after its manufacturers voluntarily reduced retail prices.
Interpretation: Evidence to date suggests that reference-based pricing of nitrates has achieved its primary goal of reducing drug expenditures. The effects of this policy on patient health, associated health care costs and administrative costs remain to be investigated.
Figures


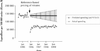
References
-
- Dickson M, Redwood H. Pharmaceutical reference prices. How do they work in practice? Pharmacoeconomics 1998;14(5):471-9. - PubMed
-
- Selke G. Reference price systems in the European Community. In: Mossialos E, Ranos C, Abel-Smith B, editors. Cost containment, pricing and financing of pharmaceuticals in the European Community: the policy makers' view. Athens: LSE Health and Pharmetrica SA; 1994. p. 147-60.
-
- Zammit-Lucia J, Dasgupta R. Reference pricing: the European experience. No. 10 in Health Policy Review Paper series, St. Mary's Hospital Medical School. London: University of London; 1995.
-
- Morton FS. The strategic response by pharmaceutical firms to the Medicaid most-favored-customer rules. Rand J Econ 1997;28(2):269-90. - PubMed
-
- Zweifel P, Crivelli L. Price regulation of drugs: lessons from Germany. J Regul Econ 1996;10:257-73.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
