Expression of Galpha 13 (Q226L) induces P19 stem cells to primitive endoderm via MEKK1, 2, or 4
- PMID: 11700306
- PMCID: PMC6007846
- DOI: 10.1074/jbc.M107031200
Expression of Galpha 13 (Q226L) induces P19 stem cells to primitive endoderm via MEKK1, 2, or 4
Abstract
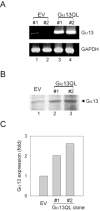
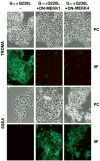
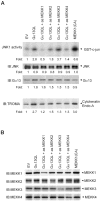
Galpha13 mediates the ability of the morphogen retinoic acid to promote primitive endoderm formation from mouse P19 embryonal carcinoma stem cells, a process that includes the obligate activation of Jun N-terminal kinase. Expression of the constitutively activated (Q226L) GTPase-deficient form of Galpha13 mimics retinoic acid and was used to investigate the signaling upstream of primitive endoderm formation. Jun N-terminal kinase 1 activity, MEK1,2, MKK4, and MEKK1 were constitutively activated in clones stably transfected to express Q226L Galpha13. Dominant negative forms of MEKK1 and MEKK4 were expressed stably in the clones harboring Q226L Galpha13. Expression of dominant negative versions of either MEKK1 or MEKK4 effectively blocks both the activation of Jun N-terminal kinase as well as the formation of primitive endoderm. Depletion of MEKK1, -2, or -4 by antisense oligodeoxynucleotides suppressed signaling from Q226L Galpha13 to JNK1 and primitive endoderm formation. We demonstrate that the signal linkage map from Galpha13 activation to primitive endoderm formation in these stem cells requires activation at three levels of the mitogen-activated protein kinase cascade: MEKK1, -2, or -4 for MAP kinase kinase kinase; MKK4 and/or MEK1 for MAP kinase kinase; and JNK1 for MAP kinase.
Figures
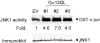

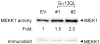



Similar articles
-
MEKK4 mediates differentiation in response to retinoic acid via activation of c-Jun N-terminal kinase in rat embryonal carcinoma P19 cells.J Biol Chem. 2000 Aug 4;275(31):24032-9. doi: 10.1074/jbc.M002747200. J Biol Chem. 2000. PMID: 10807916
-
G alpha 13 signals via p115RhoGEF cascades regulating JNK1 and primitive endoderm formation.J Biol Chem. 2004 Dec 24;279(52):54896-904. doi: 10.1074/jbc.M407581200. Epub 2004 Oct 18. J Biol Chem. 2004. PMID: 15492006
-
Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1.J Biol Chem. 1998 Oct 23;273(43):27816-23. doi: 10.1074/jbc.273.43.27816. J Biol Chem. 1998. PMID: 9774391
-
Axin utilizes distinct regions for competitive MEKK1 and MEKK4 binding and JNK activation.J Biol Chem. 2003 Sep 26;278(39):37451-8. doi: 10.1074/jbc.M305277200. Epub 2003 Jul 23. J Biol Chem. 2003. PMID: 12878610
-
JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signaling pathway.Mol Cell Biol. 1999 Nov;19(11):7539-48. doi: 10.1128/MCB.19.11.7539. Mol Cell Biol. 1999. PMID: 10523642 Free PMC article.
Cited by
-
Tumor Suppressors and Endodermal Differentiation of P19 Embryonic Stem Cells.Cell Dev Biol. 2015 Dec;4(3):e138. doi: 10.4172/2168-9296.1000e138. Epub 2015 Dec 30. Cell Dev Biol. 2015. PMID: 27413642 Free PMC article. No abstract available.
-
Ku80 is required but not sufficient for Galpha13-mediated endodermal differentiation in P19 embryonic carcinoma cells.Biochem Biophys Res Commun. 2004 Oct 8;323(1):293-8. doi: 10.1016/j.bbrc.2004.08.092. Biochem Biophys Res Commun. 2004. PMID: 15351736 Free PMC article.
-
Menin induces endodermal differentiation in aggregated P19 stem cells by modulating the retinoic acid receptors.Mol Cell Biochem. 2012 Jan;359(1-2):95-104. doi: 10.1007/s11010-011-1003-2. Epub 2011 Aug 11. Mol Cell Biochem. 2012. PMID: 21833538 Free PMC article.
-
The Grb2/Mek pathway represses Nanog in murine embryonic stem cells.Mol Cell Biol. 2006 Oct;26(20):7539-49. doi: 10.1128/MCB.00508-06. Epub 2006 Aug 14. Mol Cell Biol. 2006. PMID: 16908534 Free PMC article.
-
Puromycin-resistant lentiviral control shRNA vector, pLKO.1 induces unexpected cellular differentiation of P19 embryonic stem cells.Biochem Biophys Res Commun. 2017 Apr 29;486(2):481-485. doi: 10.1016/j.bbrc.2017.03.066. Epub 2017 Mar 16. Biochem Biophys Res Commun. 2017. PMID: 28322785 Free PMC article.
References
-
- Ip YT, Davis RJ. Curr Opin Cell Biol. 1998;10:205–219. - PubMed
-
- Morris AJ, Malbon CC. Physiol Rev. 1999;79:1373–1430. - PubMed
-
- Gao P, Malbon CC. J Biol Chem. 1996;271:9002–9008. - PubMed
-
- Crespo P, Xu N, Simonds WF, Gutkind JS. Nature. 1994;369:418–420. - PubMed
-
- Noselli S. Trends Gen. 1998;14:33–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

