Quantification of mucosal leucocyte endothelial cell interaction by in vivo fluorescence microscopy in experimental colitis in mice
- PMID: 11703368
- PMCID: PMC1906186
- DOI: 10.1046/j.1365-2249.2001.01544.x
Quantification of mucosal leucocyte endothelial cell interaction by in vivo fluorescence microscopy in experimental colitis in mice
Abstract
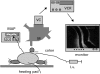
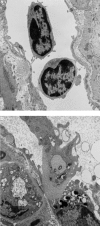
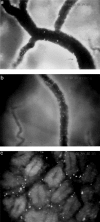
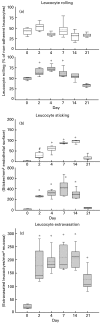
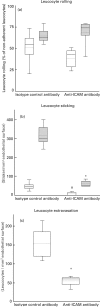
Leucocyte recruitment to sites of intestinal inflammation is a crucial, multi-step process that leads ultimately to the accumulation of cells in the inflamed tissue. We established a new in vivo model system of experimental colitis to quantify leucocyte-endothelial cell interaction and leucocyte extravasation in the inflamed mucosa of the colon. Furthermore, we investigated the pathophysiological role of ICAM-1 in the intestinal microcirculation in vivo. Using the model of dextran sodium sulphate (DSS)-induced acute and chronic colitis in mice, in vivo microscopy was performed in the colonic submucosal postcapillary venules and the submucosal collecting venules in normal or inflamed murine colonic segments. ICAM-1 expression was blocked by an anti-ICAM-1 monoclonal antibody or by suppressing NF-kappaB activation by gliotoxin. Significant increases in leucocyte adhesiveness (51-fold in postcapillary venules, 30-fold in collecting venules, P < 0.01) and extravasation (6.5-fold) could be demonstrated as early as day 2 of DSS-application in acute colitis (P < 0.01). This was paralleled by increases in both the histological damage scores and myeloperoxidase activities. In chronic dextran sodium sulphate-induced colitis significant increases in leucocyte-endothelium interactions and leucocyte extravasation were observed. Blocking ICAM-1 expression with a monoclonal antibody or gliotoxin, leucocyte sticking and extravasation were significantly down-regulated in vivo compared to controls (> 70%; P < 0.01). This new model system offers the possibility to specifically assess the role of adhesion molecules in the colonic mucosa in vivo as well as to investigate and quantify the effectiveness of experimental therapeutic approaches in acute or chronic intestinal inflammation.
Figures


References
-
- Panes J, Granger DN. Leukocyte–endothelial cell interactions. Molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–90. - PubMed
-
- Vainer B. Role of cell adhesion molecules in inflammatory bowel diseases. Scand J Gastroenterol. 1997;32:401–10. - PubMed
-
- Binion DG, West GA, Volk EE, et al. Acquired increase in leucocyte binding by intestinal microvascular endothelium in inflammatory bowel disease. Lancet. 1998;352:1742–6. - PubMed
-
- Mahler M, Bristol IJ, Leiter EH, et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol. 1998;274:G544–G551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

