Regulation of VEGF-induced endothelial cell PAF synthesis: role of p42/44 MAPK, p38 MAPK and PI3K pathways
- PMID: 11704645
- PMCID: PMC1573057
- DOI: 10.1038/sj.bjp.0704367
Regulation of VEGF-induced endothelial cell PAF synthesis: role of p42/44 MAPK, p38 MAPK and PI3K pathways
Abstract
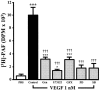
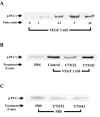
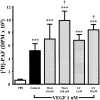
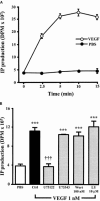
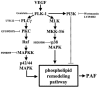
1. Vascular endothelial growth factor (VEGF) is a potent angiogenic and inflammatory mediator. We have recently shown that this latter effect requires the activation of Flk-1 receptor and subsequent endothelial cell (EC) PAF synthesis. However, the intracellular events that regulate EC PAF synthesis upon Flk-1 stimulation by VEGF remain to be elucidated. 2. Using specific inhibitors and Western blot analysis, we herein report that in bovine aortic endothelial cells (BAEC), VEGF induces the synthesis of PAF through the cascade activation of Flk-1 receptor, phospholipase Cgamma (PLCgamma), protein kinase C (PKC) and p42/44 mitogen-activated protein kinases (MAPK). 3. Moreover, we demonstrate that VEGF-mediated PAF synthesis requires the activation of p38 MAPK, likely by directing the conversion of lyso-PAF to PAF. 4. Interestingly, we observed that VEGF also promoted the activation of the phosphatidyl inositol-3-phosphate kinase (PI3K) pathway, and that its blockade potentiated PAF synthesis following a VEGF treatment. Consequently, it appears that the PI3K pathway acts as a negative regulator of EC PAF synthesis. 5. Taken together, these results allow a better understanding of the intracellular events activated upon EC stimulation by VEGF, and shed a new light on the mechanisms by which VEGF induces PAF synthesis.
Figures


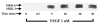


Similar articles
-
Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway.Diabetes. 1999 May;48(5):1145-55. doi: 10.2337/diabetes.48.5.1145. Diabetes. 1999. PMID: 10331422
-
Characterization of vascular endothelial growth factor's effect on the activation of protein kinase C, its isoforms, and endothelial cell growth.J Clin Invest. 1996 Nov 1;98(9):2018-26. doi: 10.1172/JCI119006. J Clin Invest. 1996. PMID: 8903320 Free PMC article.
-
Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC and PLC pathways.Br J Pharmacol. 2002 Dec;137(7):1021-30. doi: 10.1038/sj.bjp.0704956. Br J Pharmacol. 2002. PMID: 12429574 Free PMC article.
-
Signaling angiogenesis via p42/p44 MAP kinase cascade.Ann N Y Acad Sci. 2000 May;902:187-200. doi: 10.1111/j.1749-6632.2000.tb06313.x. Ann N Y Acad Sci. 2000. PMID: 10865838 Review.
-
Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells.Trends Cardiovasc Med. 2000 Nov;10(8):321-7. doi: 10.1016/s1050-1738(01)00072-x. Trends Cardiovasc Med. 2000. PMID: 11369257 Review.
Cited by
-
G protein-coupled receptors activate p38 MAPK via a non-canonical TAB1-TAB2- and TAB1-TAB3-dependent pathway in endothelial cells.J Biol Chem. 2019 Apr 12;294(15):5867-5878. doi: 10.1074/jbc.RA119.007495. Epub 2019 Feb 13. J Biol Chem. 2019. PMID: 30760523 Free PMC article.
-
Signaling in TRPV1-induced platelet activating factor (PAF) in human esophageal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2010 Feb;298(2):G233-40. doi: 10.1152/ajpgi.00409.2009. Epub 2009 Dec 3. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19959817 Free PMC article.
-
Autophagy inhibition contributes to Endostar sensitization in esophageal squamous cell carcinoma.Oncol Lett. 2017 Dec;14(6):6604-6610. doi: 10.3892/ol.2017.7017. Epub 2017 Sep 21. Oncol Lett. 2017. PMID: 29163691 Free PMC article.
-
Screening miRNAs to Hinder the Tumorigenesis of Renal Clear Cell Carcinoma Associated with KDR Expression.Curr Cancer Drug Targets. 2025;25(2):183-203. doi: 10.2174/0115680096321287240826065718. Curr Cancer Drug Targets. 2025. PMID: 39289946
-
Upregulation of microRNA-205 suppresses vascular endothelial growth factor expression-mediated PI3K/Akt signaling transduction in human keloid fibroblasts.Exp Biol Med (Maywood). 2017 Feb;242(3):275-285. doi: 10.1177/1535370216669839. Epub 2016 Oct 4. Exp Biol Med (Maywood). 2017. Retraction in: Exp Biol Med (Maywood). 2023 Dec;248(23):2493. doi: 10.1177/15353702221144939. PMID: 27651436 Free PMC article. Retracted.
References
-
- BERNATCHEZ P.N., SOKER S., SIROIS M.G. VEGF effect on endothelial cell proliferation migration and PAF synthesis is mediated through the activation of Flk-1 receptor. J. Biol. Chem. 1999;274:31047–31054. - PubMed
-
- BLIGH E.G., DYER W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

