Group A streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors
- PMID: 11705914
- PMCID: PMC98828
- DOI: 10.1128/IAI.69.12.7402-7412.2001
Group A streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors
Abstract
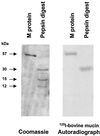
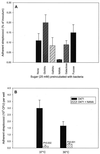
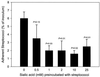
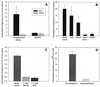
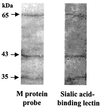
The first step in the colonization of group A streptococci (Streptococcus pyogenes) is adherence to pharyngeal epithelial cells. Prior to adherence to their target tissue, the first barrier that the streptococci encounter is the mucous layer of the respiratory tract. The present study was undertaken to characterize the interaction between mucin, the major glycoprotein component of mucus, and streptococci. We report here that S. pyogenes is able to bind to bovine submaxillary mucin in solid-phase microtiter plate assays. Western blots probed with (125)I-labeled mucin and a panel of monoclonal antibodies revealed that the streptococcal M protein is one of two cell wall-associated proteins responsible for this binding. The binding was further localized to the N-terminal portion of the M molecule. Further analysis revealed that the M protein binds to the sialic acid moieties on mucin, and this interaction seems to be based on M-protein conformation rather than specific amino acid sequences. We found that sialic acid also plays a critical role in the adherence of an M6 streptococcal strain to the Detroit 562 human pharyngeal cell line and have identified alpha2-6-linked sialic acid as an important sialylated linkage for M-protein recognition. Western blot analysis of extracted pharyngeal cell membrane proteins identified three potential sialic acid-containing receptors for the M protein. The results are the first to show that sialic acid not only is involved in the binding of the streptococci to mucin but also plays an important role in adherence of group A streptococci to the pharyngeal cell surface.
Figures


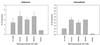
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

