Identification, characterization, and variation in expression of two serologically distinct O-antigen epitopes in lipopolysaccharides of Campylobacter fetus serotype A strains
- PMID: 11705938
- PMCID: PMC98852
- DOI: 10.1128/IAI.69.12.7596-7602.2001
Identification, characterization, and variation in expression of two serologically distinct O-antigen epitopes in lipopolysaccharides of Campylobacter fetus serotype A strains
Abstract
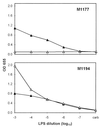
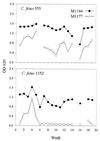
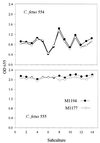
Monoclonal antibodies (MAbs) to the lipopolysaccharide (LPS) O-antigens of Campylobacter fetus serotype A and B strains were produced. Eight MAbs specific for serotype A LPS were characterized on immunoblots of C. fetus serotype A LPS. Two immunoblot patterns were observed and were used to divide the eight MAbs into two groups. MAbs M1177 and M1194 were selected as representative of the two groups and were used in an enzyme-linked immunosorbent assay (ELISA) to examine the LPS O-antigen epitopes of 37 serotype A C. fetus subsp. fetus and C. fetus subsp. venerealis strains. Thirty-three strains (89%) reacted with both M1177 and M1194, 2 strains reacted only with M1177, and 2 strains reacted only with M1194. To further characterize the O-antigen epitopes, purified serotype A LPS was treated using various temperature and pH conditions and the effect of the treatments on the reactivity of the LPS with MAbs M1177 and M1194 was evaluated by ELISA. While no difference among several treatments was observed, heating serotype A LPS under alkaline conditions decreased the reaction with M1177 to background levels and increased the reaction with M1194. MAbs M1177 and M1194 were also used with ELISA to investigate in vivo and in vitro expression of the two O-antigen epitopes. There was substantial variation in expression of the two epitopes among weekly isolates of two C. fetus serotype A strains recovered from experimentally infected heifers. There was minimal variation in expression of the two epitopes in successive subcultures of three C. fetus serotype A strains.
Figures


Similar articles
-
Validation of a monoclonal antibody-based capture enzyme-linked immunosorbent assay for detection of Campylobacter fetus.Clin Diagn Lab Immunol. 2005 Nov;12(11):1261-8. doi: 10.1128/CDLI.12.11.1261-1268.2005. Clin Diagn Lab Immunol. 2005. PMID: 16275938 Free PMC article.
-
Evaluation of a monoclonal antibody-based enzyme-linked immunosorbent assay for detection of Campylobacter fetus in bovine preputial washing and vaginal mucus samples.Vet Microbiol. 2004 Oct 5;103(1-2):77-84. doi: 10.1016/j.vetmic.2004.07.008. Vet Microbiol. 2004. PMID: 15381269
-
Monoclonal antibodies specific for Campylobacter fetus lipopolysaccharides.Vet Microbiol. 2002 Jun 5;87(1):37-49. doi: 10.1016/s0378-1135(02)00026-3. Vet Microbiol. 2002. PMID: 12079745
-
Antigenic and restriction enzyme analysis of isolates of Campylobacter fetus subsp venerealis recovered from persistently infected cattle.Am J Vet Res. 1989 Jun;50(6):807-13. Am J Vet Res. 1989. PMID: 2548417
-
Electrophoretic and immunoblot analysis of Campylobacter fetus lipopolysaccharides.Vet Microbiol. 1996 Jul;51(1-2):105-14. doi: 10.1016/0378-1135(96)00015-6. Vet Microbiol. 1996. PMID: 8828127
Cited by
-
Validation of a monoclonal antibody-based capture enzyme-linked immunosorbent assay for detection of Campylobacter fetus.Clin Diagn Lab Immunol. 2005 Nov;12(11):1261-8. doi: 10.1128/CDLI.12.11.1261-1268.2005. Clin Diagn Lab Immunol. 2005. PMID: 16275938 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

