The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice
- PMID: 11705952
- PMCID: PMC98866
- DOI: 10.1128/IAI.69.12.7711-7717.2001
The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice
Abstract
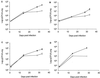
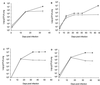
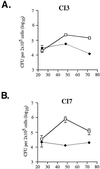
Murine macrophages effect potent antimycobacterial function via the production of nitric oxide by the inducible isoform of the enzyme nitric oxide synthase (NOS2). The protective role of reactive nitrogen intermediates (RNI) against Mycobacterium tuberculosis infection has been well established in various murine experimental tuberculosis models using laboratory strains of the tubercle bacillus to establish infection by the intravenous route. However, important questions remain about the in vivo importance of RNI in host defense against M. tuberculosis. There is some evidence that RNI play a lesser role following aerogenic, rather than intravenous, M. tuberculosis infection of mice. Furthermore, in vitro studies have demonstrated that different strains of M. tuberculosis, including clinical isolates, vary widely in their susceptibility to the antimycobacterial effects of RNI. Thus, we sought to test rigorously the protective role of RNI against infection with recent clinical isolates of M. tuberculosis following both aerogenic and intravenous challenges. Three recently isolated and unique M. tuberculosis strains were used to infect both wild-type (wt) C57BL/6 and NOS2 gene-disrupted mice. Regardless of the route of infection, NOS2(-/-) mice were much more susceptible than wt mice to any of the clinical isolates or to either the Erdman or H37Rv laboratory strain of M. tuberculosis. Mycobacteria replicated to much higher levels in the organs of NOS2(-/-) mice than in those of wt mice. Although the clinical isolates all exhibited enhanced virulence in NOS2(-/-) mice, they displayed distinct growth rates in vivo. The present study has provided results indicating that RNI are required for the control of murine tuberculous infection caused by both laboratory and clinical strains of M. tuberculosis. This protective role of RNI is essential for the control of infection established by either intravenous or aerogenic challenge.
Figures
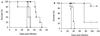
Similar articles
-
The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis.Cell Microbiol. 2003 Sep;5(9):637-48. doi: 10.1046/j.1462-5822.2003.00307.x. Cell Microbiol. 2003. PMID: 12925133
-
The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response.Infect Immun. 2006 Dec;74(12):6855-64. doi: 10.1128/IAI.01022-06. Epub 2006 Oct 9. Infect Immun. 2006. PMID: 17030572 Free PMC article.
-
Susceptibility of germfree phagocyte oxidase- and nitric oxide synthase 2-deficient mice, defective in the production of reactive metabolites of both oxygen and nitrogen, to mucosal and systemic candidiasis of endogenous origin.Infect Immun. 2005 Mar;73(3):1313-20. doi: 10.1128/IAI.73.3.1313-1320.2005. Infect Immun. 2005. PMID: 15731028 Free PMC article.
-
The role of nitric oxide in the immune response of tuberculosis.J Korean Med Sci. 1997 Dec;12(6):481-7. doi: 10.3346/jkms.1997.12.6.481. J Korean Med Sci. 1997. PMID: 9443084 Free PMC article. Review.
-
[Nontuberculous mycobacteriosis; the present status and in the future. Mechanisms of host resistance to Mycobacterium avium complex and Mycobacterium tuberculosis infection].Kekkaku. 1998 Feb;73(2):71-6. Kekkaku. 1998. PMID: 9545699 Review. Japanese.
Cited by
-
The Interplay between Mycobacterium tuberculosis and Human Microbiome.Clin Pract. 2024 Jan 24;14(1):198-213. doi: 10.3390/clinpract14010017. Clin Pract. 2024. PMID: 38391403 Free PMC article. Review.
-
NOS2/miR-493-5p Signaling Regulates in the LPS-Induced Inflammatory Response in the RAW264.7 Cells.Biochem Genet. 2023 Jun;61(3):1097-1112. doi: 10.1007/s10528-022-10297-2. Epub 2022 Nov 30. Biochem Genet. 2023. PMID: 36449151
-
How mycobacterium tuberculosis infection could lead to the increasing risks of chronic fatigue syndrome and the potential immunological effects: a population-based retrospective cohort study.J Transl Med. 2022 Feb 21;20(1):99. doi: 10.1186/s12967-022-03301-1. J Transl Med. 2022. PMID: 35189895 Free PMC article.
-
Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide.Infect Immun. 2003 Mar;71(3):1442-52. doi: 10.1128/IAI.71.3.1442-1452.2003. Infect Immun. 2003. PMID: 12595462 Free PMC article.
-
Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide Synthase 2-independent defense in mice.J Exp Med. 2002 Oct 7;196(7):991-8. doi: 10.1084/jem.20021186. J Exp Med. 2002. PMID: 12370260 Free PMC article.
References
-
- Alland D, Kalkut G E, Moss A R, McAdam R A, Hahn J A, Bosworth W, Drucker E, Bloom B R. Transmission of tuberculosis in New York City, an analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. - PubMed
-
- Chan J, Flynn J L. Nitric oxide in Mycobacterium tuberculosis infection. In: Fang F, editor. Nitric oxide and infection. New York. N.Y: Plenum Publishers; 1999. pp. 281–310.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

