Role of complement in Mycobacterium avium pathogenesis: in vivo and in vitro analyses of the host response to infection in the absence of complement component C3
- PMID: 11705954
- PMCID: PMC98868
- DOI: 10.1128/IAI.69.12.7729-7735.2001
Role of complement in Mycobacterium avium pathogenesis: in vivo and in vitro analyses of the host response to infection in the absence of complement component C3
Abstract
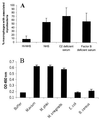
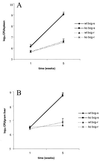
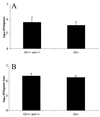
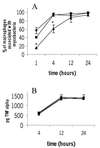
We investigated the importance of the host complement system in the pathogenesis of disease mediated by the intramacrophage pathogen Mycobacterium avium. Mycobacteria opsonized with complement are efficiently ingested by macrophages through various complement receptors. Furthermore, unlike other bacteria, mycobacteria can activate both the alternative and classical complement pathways in the absence of specific antibodies. Therefore, to examine the role of complement in the mycobacterial infection process in vivo, mice deficient in complement component C3 were infected with M. avium. Surprisingly, C3-deficient mice infected intravenously with M. avium displayed no difference in bacterial burden or granulomatous response compared to wild-type control mice. C3-sufficient mice and C3-deficient mice were equally susceptible to infection by M. avium regardless of the genotype at the bcg locus, a locus known to confer susceptibility to infection with intracellular pathogens. In vitro studies using mouse bone marrow-derived macrophages resulted in significant M. avium invasion of macrophages in the absence of C3; however, the kinetics of infection were delayed compared to complement-mediated invasion. The data indicate that complement does not play an essential role in mediating M. avium infections in the mouse and suggest either that other invasion mechanisms can compensate for the absence of complement-mediated entry or that complement is not a major mycobacterial opsonin in vivo.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

