Chlamydia pneumoniae infects and multiplies in lymphocytes in vitro
- PMID: 11705957
- PMCID: PMC98871
- DOI: 10.1128/IAI.69.12.7753-7759.2001
Chlamydia pneumoniae infects and multiplies in lymphocytes in vitro
Abstract
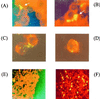
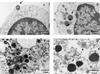
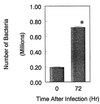
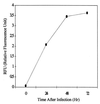
The obligate intracellular pathogen Chlamydia (Chlamydophila) pneumoniae is known to be associated with some chronic inflammatory diseases, such as atherosclerosis. Interaction between C. pneumoniae and immune cells is important in the development of such diseases. However, susceptibility of immune cells, particularly lymphocytes, to C. pneumoniae infection has not been reported, even though lymphocytes play a pivotal role in the development of the diseases caused by this bacterium. In this regard, we examined the susceptibility of lymphocytes to C. pneumoniae infection in vitro. The results demonstrated that human peripheral blood lymphocytes as well as mouse spleen lymphocytes could be infected with C. pneumoniae. Furthermore, purified T lymphocytes as well as established T-lymphocyte cell line cells showed an obvious susceptibility to C. pneumoniae infection, indicating that T cells could be one of the host cells for this bacterial infection. These findings reveal a new infection site for C. pneumoniae, i.e., lymphocytes.
Figures

Similar articles
-
Inhibition of lymphocyte CD3 expression by Chlamydophila pneumoniae infection.Microb Pathog. 2008 Oct;45(4):290-6. doi: 10.1016/j.micpath.2008.06.005. Epub 2008 Jul 12. Microb Pathog. 2008. PMID: 18674609
-
Identification of an in vivo CD4+ T cell-mediated response to polymorphic membrane proteins of Chlamydia pneumoniae during experimental infection.FEMS Immunol Med Microbiol. 2004 Mar 8;40(2):129-37. doi: 10.1016/S0928-8244(03)00300-6. FEMS Immunol Med Microbiol. 2004. PMID: 14987731
-
Chlamydia pneumoniae infection suppresses Staphylococcus enterotoxin B-induced proliferation associated with down-expression of CD25 in lymphocytes.Can J Microbiol. 2010 Apr;56(4):289-94. doi: 10.1139/w10-018. Can J Microbiol. 2010. PMID: 20453895
-
Cell mediated immunity to Chlamydia pneumoniae.Scand J Infect Dis Suppl. 1997;104:18-21. Scand J Infect Dis Suppl. 1997. PMID: 9259075 Review.
-
Chlamydia pneumoniae infections in mouse models: relevance for atherosclerosis research.Cardiovasc Res. 2005 Feb 1;65(2):317-27. doi: 10.1016/j.cardiores.2004.09.031. Cardiovasc Res. 2005. PMID: 15639470 Review.
Cited by
-
Chlamydia pneumoniae infection of monocytes in vitro stimulates innate and adaptive immune responses relevant to those in Alzheimer's disease.J Neuroinflammation. 2014 Dec 24;11:217. doi: 10.1186/s12974-014-0217-0. J Neuroinflammation. 2014. PMID: 25540075 Free PMC article.
-
Detection of Chlamydia in the peripheral blood cells of normal donors using in vitro culture, immunofluorescence microscopy and flow cytometry techniques.BMC Infect Dis. 2006 Feb 10;6:23. doi: 10.1186/1471-2334-6-23. BMC Infect Dis. 2006. PMID: 16472397 Free PMC article.
-
Chlamydia pneumoniae growth inhibition in cells by the steroid receptor antagonist RU486 (mifepristone).Antimicrob Agents Chemother. 2008 Jun;52(6):1991-8. doi: 10.1128/AAC.01416-07. Epub 2008 Mar 17. Antimicrob Agents Chemother. 2008. PMID: 18347111 Free PMC article.
-
Novel Parachlamydia acanthamoebae quantification method based on coculture with amoebae.Appl Environ Microbiol. 2008 Oct;74(20):6397-404. doi: 10.1128/AEM.00841-08. Epub 2008 Aug 29. Appl Environ Microbiol. 2008. PMID: 18757579 Free PMC article.
-
Involvement of nicotinic acetylcholine receptors in controlling Chlamydia pneumoniae growth in epithelial HEp-2 cells.Infect Immun. 2003 Jun;71(6):3645-7. doi: 10.1128/IAI.71.6.3645-3647.2003. Infect Immun. 2003. PMID: 12761154 Free PMC article.
References
-
- Fryer R H, Woods M L, Rodgers G M. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Investig Med. 1994;45:168–174. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

