Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas
- PMID: 11705964
- PMCID: PMC98878
- DOI: 10.1128/IAI.69.12.7820-7831.2001
Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas
Abstract
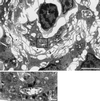
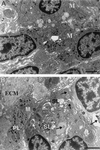
Mycobacterium marinum causes long-term subclinical granulomatous infection in immunocompetent leopard frogs (Rana pipiens). These granulomas, organized collections of activated macrophages, share many morphological features with persistent human tuberculous infection. We examined organs of frogs with chronic M. marinum infection using transmission electron microscopy in conjunction with immunohistochemistry and acid phosphatase cytochemistry to better define the bacterium-host interplay during persistent infection. Bacteria were always found within macrophage phagosomes. These phagosomes were often fused to lysosomes, in sharp contrast to those formed during in vitro infection of J774 macrophage-like cells by M. marinum. The infected macrophages in frog granulomas showed various levels of activation, as evidenced by morphological changes, including epithelioid transformation, recent phagocytic events, phagolysosomal fusion, and disintegration of bacteria. Our results demonstrate that even long-term granulomas are dynamic environments with regard to the level of host cell activation and bacterial turnover and suggest a continuum between constantly replicating bacteria and phagocytic killing that maintains relatively constant bacterial numbers despite an established immune response. Infection with a mutant bacterial strain with a reduced capacity for intracellular replication shifted the balance, leading to a greatly reduced bacterial burden and inflammatory foci that differed from typical granulomas.
Figures








References
-
- Ando M, Dannenberg A M., Jr Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. IV. Macrophage turnover, lysosomal enzymes, and division in healing lesions. Lab Investig. 1972;27:466–472. - PubMed
-
- Ando M, Dannenberg A M, Jr, Shima K. Macrophage accumulation, division, maturation and digestive and microbicidal capacities in tuberculous lesions. II. Rate at which mononuclear cells enter and divide in primary BCG lesions and those of reinfection. J Immunol. 1972;109:8–19. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

