Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud
- PMID: 11706050
- PMCID: PMC2198861
- DOI: 10.1083/jcb.200106065
Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud
Abstract
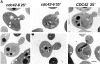
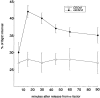
The Rho family GTPase Cdc42 is a key regulator of cell polarity and cytoskeletal organization in eukaryotic cells. In yeast, the role of Cdc42 in polarization of cell growth includes polarization of the actin cytoskeleton, which delivers secretory vesicles to growth sites at the plasma membrane. We now describe a novel temperature-sensitive mutant, cdc42-6, that reveals a role for Cdc42 in docking and fusion of secretory vesicles that is independent of its role in actin polarization. cdc42-6 mutants can polarize actin and deliver secretory vesicles to the bud, but fail to fuse those vesicles with the plasma membrane. This defect is manifested only during the early stages of bud formation when growth is most highly polarized, and appears to reflect a requirement for Cdc42 to maintain maximally active exocytic machinery at sites of high vesicle throughput. Extensive genetic interactions between cdc42-6 and mutations in exocytic components support this hypothesis, and indicate a functional overlap with Rho3, which also regulates both actin organization and exocytosis. Localization data suggest that the defect in cdc42-6 cells is not at the level of the localization of the exocytic apparatus. Rather, we suggest that Cdc42 acts as an allosteric regulator of the vesicle docking and fusion apparatus to provide maximal function at sites of polarized growth.
Figures








References
-
- Brennwald, P., and P. Novick. 1993. Interactions of three domains distinguishing the Ras-related GTP-binding proteins Ypt1 and Sec4. Nature. 362:560–563. - PubMed
-
- Brennwald, P., B. Kearns, K. Champion, S. Keranen, V. Bankaitis, and P. Novick. 1994. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 79:245–258. - PubMed
-
- Drubin, D.G., and W.J. Nelson. 1996. Origins of cell polarity. Cell. 84:335–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

