Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells
- PMID: 11706053
- PMCID: PMC2198875
- DOI: 10.1083/jcb.200108080
Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells
Abstract
Efficient phagocytosis of apoptotic cells is important for normal tissue development, homeostasis, and the resolution of inflammation. Although many receptors have been implicated in the clearance of apoptotic cells, the roles of these receptors in the engulfment process have not been well defined. We developed a novel system to distinguish between receptors involved in tethering of apoptotic cells versus those inducing their uptake. Our results suggest that regardless of the receptors engaged on the phagocyte, ingestion does not occur in the absence of phosphatidylserine (PS). Further, recognition of PS was found to be dependent on the presence of the PS receptor (PSR). Both PS and anti-PSR antibodies stimulated membrane ruffling, vesicle formation, and "bystander" uptake of cells bound to the surface of the phagocyte. We propose that the phagocytosis of apoptotic cells requires two events: tethering followed by PS-stimulated, PSR-mediated macropinocytosis.
Figures









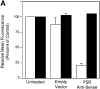


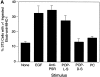



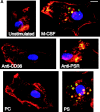

Comment in
-
Tethering and tickling: a new role for the phosphatidylserine receptor.J Cell Biol. 2001 Nov 12;155(4):501-4. doi: 10.1083/jcb.200110066. Epub 2001 Nov 12. J Cell Biol. 2001. PMID: 11706046 Free PMC article. Review.
References
-
- Albert, M.L., J.I. Kim, and R.B. Birge. 2000. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2:899–905. - PubMed
-
- Balasubramanian, K., and A.J. Schroit. 1998. Characterization of phosphatidylserine-dependent beta2-glycoprotein I macrophage interactions. Implications for apoptotic cell clearance by phagocytes. J. Biol. Chem. 273:29272–29277. - PubMed
-
- Balasubramanian, K., J. Chandra, and A.J. Schroit. 1997. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition. J. Biol. Chem. 272:31113–31117. - PubMed
-
- Bretscher, M.S., and C. Aguado-Velasco. 1998. Membrane traffic during cell locomotion. Curr. Opin. Cell Biol. 10:537–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

